7.已知下列几种烷烃的燃烧热数据如下:
|
烷烃 |
甲烷 |
乙烷 |
丙烷 |
丁烷 |
|
燃烧热/(kJ/mol-1) |
890 |
1560 |
2220 |
2880 |
据此判断,表示戊烷燃烧的热化学方程式正确的是
A.C5H12(l) + 8O2(g) = 5CO2(g) + 6H2O(l);△H = - 3540 kJ/mol
B.C5H12(l) + 8O2(g) = 5CO2(g) + 6H2O(g);△H = - 3540 kJ/mol
C.C5H12(l) + 8O2(g) = 5CO2(g) + 6H2O(g);△H = + 3540 kJ/mol
D.C5H12(l) + 8O2(g) = 5CO2(g) + 6H2O(l);△H = + 3540 kJ/mol

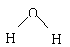
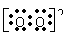
 C.Na2O2的电子式为Na+
Na+
C.Na2O2的电子式为Na+
Na+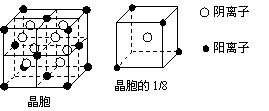 (1)A和E所形成的化合物的电子式是
(1)A和E所形成的化合物的电子式是
 (10分)抗禽流感药物达菲是以莽草酸(A)为原料合成制得。莽草酸可以从香料“八角”中提取,其结构简式如下:
(10分)抗禽流感药物达菲是以莽草酸(A)为原料合成制得。莽草酸可以从香料“八角”中提取,其结构简式如下: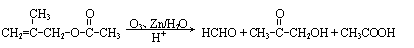

 (10分)某市拟投资建设一个工业酒精厂,目的是用工业酒精与汽油混合制成“乙醇汽油”,以节省石油资源。已知制酒精的方法有三种:
(10分)某市拟投资建设一个工业酒精厂,目的是用工业酒精与汽油混合制成“乙醇汽油”,以节省石油资源。已知制酒精的方法有三种:
 ①在催化剂作用下乙烯与水反应; ②CH3CH2Br
+ H2O CH3CH2OH
+ HBr
①在催化剂作用下乙烯与水反应; ②CH3CH2Br
+ H2O CH3CH2OH
+ HBr