��Ŀ����
12������˵����ȷ���ǣ�������| A�� | ��1ml 1mol/L��NaOH����Һ�еμ�1��2��2mol/LMgCl2��Һ�а�ɫ�������ɣ��ٵμ�2��0.1mol/LFeCl3��Һ�����ֺ��ɫ��������֤��Mg��OH��2��Ksp����Fe��OH��3 | |
| B�� | ��pH=3��CH3COOH��Һ��pH=11��NaOH��Һ�������Ϻ����Һ������ | |
| C�� | ��0.1mol/L��CH3COOH��Һ��0.1mol/L��NaOH��Һ�������Ϻ����Һ������ | |
| D�� | ij���ʵ���Һ����ˮ�������c��H+��=1��10-a mol/L����a��7�������Һ��pHһ��Ϊ14-a |
���� A������������Һ����������������Ũ�ȴ�ʹþ���ӡ������ӳ�����
B��pH=11��NaOH��ҺŨ��Ϊ0.001mol/L��������������ʣ�pH=3��CH3COOH��ҺŨ��Զ����0.001mol/L���������ϵõ�CH3COOH��CH3COONa�����Һ����CH3COOH��Ũ��Զ����CH3COONa��Ũ�ȣ�
C��ǡ�÷�Ӧ�õ�CH3COONa��Һ�������ˮ�⣬��Һ�ʼ��ԣ�
D��ij���ʵ���Һ����ˮ�������c��H+��=1��10-a mol/L����a��7����Һ�ʼ��ԣ�����Ϊ�����Һ��Ҳ����Ϊ��ˮ��ʼ��ԣ�
��� �⣺A������������Һ����������������Ũ�ȴ�ʹþ���ӡ������ӳ�����������������ת�������ܱȽ�Mg��OH��2��Fe��OH��3���ܽ�ȣ���A����
B��pH=11��NaOH��ҺŨ��Ϊ0.001mol/L��������������ʣ�pH=3��CH3COOH��ҺŨ��Զ����0.001mol/L���������ϵõ�CH3COOH��CH3COONa�����Һ����CH3COOH��Ũ��Զ����CH3COONa��Ũ�ȣ�����̶ȴ���ˮ��̶ȣ���Һ�����ԣ���B��ȷ��
C��ǡ�÷�Ӧ�õ�CH3COONa��Һ��Ϊǿ�������Σ������ˮ�⣬�ƻ�ˮ�ĵ���ƽ�⣬��Һ�ʼ��ԣ���C����
D��ij���ʵ���Һ����ˮ�������c��H+��=1��10-a mol/L����a��7����Һ�ʼ��ԣ�����Ϊ�����Һ����ʱ��ҺpH=a��Ҳ����Ϊ��ˮ��ʼ��ԣ���ʱ��ҺpH=14-a����D����
��ѡ��B��
���� ���⿼����Һ������ж���pH���㡢����ˮ�⡢������ʵ��롢�ܶȻ��ȣ�ע����Һ�������pH��ϵ��D����Һ��������Ũ����ˮ����������Ũ�Ȳ�ͬ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�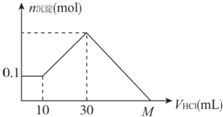 ijһ�������ʿ�����NaOH��AlCl3��MgCl2�еļ�����ɣ�����һ��������ˮ���г�������������������Һ����μ���5 mol/L�����ᣬͼ��ʾ�����������������ı仯��ϵ��ͼ��M���ʾ�Ѽ���������������M�������ǣ�������
ijһ�������ʿ�����NaOH��AlCl3��MgCl2�еļ�����ɣ�����һ��������ˮ���г�������������������Һ����μ���5 mol/L�����ᣬͼ��ʾ�����������������ı仯��ϵ��ͼ��M���ʾ�Ѽ���������������M�������ǣ�������| A�� | 70 mL | B�� | 90 mL | C�� | 100 mL | D�� | 130 mL |
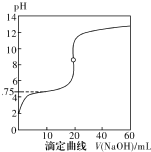 25��ʱ����0.1000mol•L-1NaOH��Һ�ζ�20mL0.1000mol•L-1һԪ��HA��pKa=-lgKa=4.75����Һ����ζ���������ͼ��ʾ������˵����ȷ���ǣ�������
25��ʱ����0.1000mol•L-1NaOH��Һ�ζ�20mL0.1000mol•L-1һԪ��HA��pKa=-lgKa=4.75����Һ����ζ���������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ������40mLNaOH��Һʱ����Һ�У�c��Na+����c��A-����c��OH-����c��H+�� | |
| B�� | ����Һ��c��H+��+c��OH-��=2��10-7ʱ��c��Na+����c��A-����c��OH-��=c��H+�� | |
| C�� | ������NaOH��Һ20mLʱc��OH-����c��H+��+c��HA�� | |
| D�� | ���ζ���pH=4.75ʱ��c��A-��=c��HA�� |
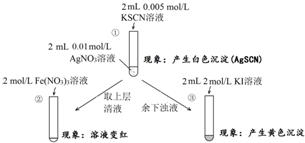
| A�� | ����Һ�д���ƽ�⣺AgSCN��s��?Ag+��aq��+SCN-��aq�� | |
| B�� | ������ɫ�仯˵���ϲ���Һ�к���SCN- | |
| C�� | ������ɫ�仯˵����AgI���� | |
| D�� | ��ʵ�����֤��AgI��AgSCN������ |
| A�� | pH=2��HA��Һ��pH=12��MOH��Һ����Ȼ�ϣ�c��H+��+c��M+��=c��OH-��+c��A-�� | |
| B�� | B��ˮ�������Ϻ���Һ�����ԣ���Һ�п��ܴ��ڣ�c��NH4+����c��Cl-�� | |
| C�� | ��һ���¶��£���ͬpH���������������Һ��ˮ���������c��H+���ֱ���1.0��0��10-amol/L����1.0��10-bmol/L���ڴ��¶�ʱ��ˮ�����ӻ�Ϊ1.0��10-��a+b�� | |
| D�� | �����£�0.1mol/L��HA pH=3��0.1mol/L ��BOH pH=13������BA��Һ��pH��7 |
| ���������� | ����������+�������� | ʧˮ������������+�������� |
| 11.70g | 14.2g | 13.2g |
| A�� | �����к��в��ֽ������ | B�� | û�з��ڸ���������ȴ | ||
| C�� | ʵ��ǰ�������������ˮ | D�� | û�н��к��ز��� |
��HF��aq��+OH-��aq��?H2O��l��+F��aq����H=-67.7kJ/mol
��H+��aq��+OH-��aq���TH2O��l����H=-57.3kJ/mol
��20Ml0��lmol/L��������еμ�0��lmol/L��NaOH V mL������˵����ȷ���ǣ�������
| A�� | �����ĵ��뷽��ʽ����ЧӦ�ɱ�ʾΪ��HF��aq��?H+��aq��+F-��aq����H=+10.4kJ/mol | |
| B�� | ��V=20 mLʱ����Һ�У�c��OH-��=c��HF��+c��H+�� | |
| C�� | ��V=20 mLʱ����Һ�У�c��F-��=c��Na+��=0.1mol/L | |
| D�� | ��v��0ʱ����Һ��һ������c��Na+����c��F-����c��OH-����c��H+�� |
| ���� | Fe��OH��3 | Cu��OH��2 | Zn��OH��2 | CuS | ZnS |
| Ksp | 4.0��10-38 | 5.0��10-20 | 2.0��10-16 | 8.5��10-45 | 1.2��10-23 |
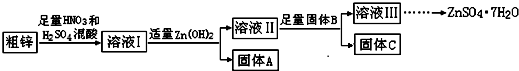
��1������A����Ҫ�ɷ���Fe��OH��3���������B����Ҫ�����ǽ�Cu2+����ͭ������Һ���з��������
��2����п�е�ͭ��ϡ������Һ��Ӧ�����ӷ���ʽΪ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
��3������ҺII��c��Cu2+��Ϊ0.05mol•L-1������ҺII��pH��5��
��4����B��Zn��ȡ8.320gC��ȫ�ܽ���500mL 1mol•L-1ϡ�����У����ռ���2240mL���壬����������Һ�м���NaOH��Һ���պ����ɳ�����࣬��ʱ���ó���������m����ȡֵ��Χ��14.7��14.85g����B����һ�����ʣ�ȡ����C���Թ��У����������������г�����ζ���壬��÷�Ӧ�����ӷ���ʽΪZnS+2H+=Zn2++H2S����
��5����ҺIII�������루NH4��2S��Һ��Ӧ�Ʊ�ZnS��ʵ��������ѡ�ã�NH4��2S��Һ������Na2S��Һ��Ϊ��Ӧ�����Ϊ�����Ƶõ�ZnS�лẬ�н϶��Zn��OH��2���ʣ�
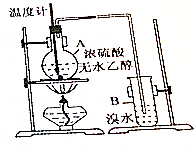 ��֪�Ҵ���Ũ������ȵ�170��ʱ��������ϩ��
��֪�Ҵ���Ũ������ȵ�170��ʱ��������ϩ��