��Ŀ����
2�����ڽ���п�����ļ�ֵ���ߣ����ҹ���ҵп���ϵĻ��������ʱȽϵͣ�ij�������о����ú�����ͭ�����Ĵ�п�Ʊ�����п��������ʵ���Դ�ۺ����ã��乤������ͼ��ͼ�м�������ʾ�Ϊ���������й��������£�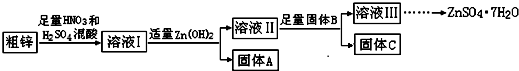
| ���� | Fe��OH��3 | Cu��OH��2 | Zn��OH��2 | CuS | ZnS |
| Ksp | 4.0��10-38 | 5.0��10-20 | 2.0��10-16 | 8.5��10-45 | 1.2��10-23 |
��1������A����Ҫ�ɷ���Fe��OH��3���������B����Ҫ�����ǽ�Cu2+����ͭ������Һ���з��������
��2����п�е�ͭ��ϡ������Һ��Ӧ�����ӷ���ʽΪ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
��3������ҺII��c��Cu2+��Ϊ0.05mol•L-1������ҺII��pH��5��
��4����B��Zn��ȡ8.320gC��ȫ�ܽ���500mL 1mol•L-1ϡ�����У����ռ���2240mL���壬����������Һ�м���NaOH��Һ���պ����ɳ�����࣬��ʱ���ó���������m����ȡֵ��Χ��14.7��14.85g����B����һ�����ʣ�ȡ����C���Թ��У����������������г�����ζ���壬��÷�Ӧ�����ӷ���ʽΪZnS+2H+=Zn2++H2S����
��5����ҺIII�������루NH4��2S��Һ��Ӧ�Ʊ�ZnS��ʵ��������ѡ�ã�NH4��2S��Һ������Na2S��Һ��Ϊ��Ӧ�����Ϊ�����Ƶõ�ZnS�лẬ�н϶��Zn��OH��2���ʣ�
���� ���ú�ͭ�����Ĵ�п�Ʊ�����п����ͭ��ͭ���������ᡢ���ᷴӦ������ͭ���ӡ������ӣ�����Ksp��֪��������ͭ��������п�����ܣ�����Һ�м���Zn��OH��2���õ�����AΪFe��OH��3��������������B����B��ͬ����C�ɷֲ�ͬ������Һ��Ϊ����п��Һ���Ʊ���ˮ������п����ֹ�ᾧˮʧˮ������ȴ�ᾧ�����ˡ�ϴ�ӡ�������õ��ϴ���������п���壻
��1���������Ϸ�������ɷ�����֪Ҫ����ͭ���ӣ�
��2��Cu�����ᷴӦ��������ͭ��NO��ˮ��
��3�������ܶȻ�����������Һ�е�����������Ũ�ȣ��ټ���pH��
��4����B��Zn�������CΪZn��Cu�Ļ����ɹ�ϵʽZn��Cu��2e-��Zn2+��Cu2+��2OH-��Cu��OH��2��Zn��OH��2��֪���������ӵ����ʵ����������������������
��5��Na2S����Һ��ˮ���Լ��ԣ�
��� �⣺���ú�ͭ�����Ĵ�п�Ʊ�����п����ͭ��ͭ���������ᡢ���ᷴӦ������ͭ���ӡ������ӣ�����Ksp��֪��������ͭ��������п�����ܣ�����Һ�м���Zn��OH��2���õ�����AΪFe��OH��3��������������B����B��ͬ����C�ɷֲ�ͬ������Һ��Ϊ����п��Һ���Ʊ���ˮ������п����ֹ�ᾧˮʧˮ������ȴ�ᾧ�����ˡ�ϴ�ӡ�������õ��ϴ���������п���壬
��1�������Ϸ�����֪����A����Ҫ�ɷ���Fe��OH��3������B��Ϊ������ͭ������ͭ����п��п�ȣ����ڳ�ȥ��Һ���е�Cu2+��
�ʴ�Ϊ��Fe��OH��3����ȥ��Һ���е�Cu2+��
��2����п�е�ͭ��ϡ������Һ��Ӧ�����ӷ���ʽΪ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O���ʴ�Ϊ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
��3������Һ����c��Cu2+��Ϊ0.05mol•L-1����Ksp=5.0��10-20��֪��c��OH-��=$\sqrt{\frac{5��1{0}^{-20}}{0.05}}$mol/L=1.0��10-9mol/L����c��H+��=1.0��10-5mol/L��
����pH��5��
�ʴ�Ϊ��5��
��4����B��Zn�������CΪZn��Cu�Ļ���ȡ8.320gC��ȫ�ܽ���500mL1mol•L-1ϡ�����У����ռ���2240mL���壬��n��NO��=$\frac{2.24L}{22.4L/mol}$=0.1mol��ת��0.3mol���ӣ��ɹ�ϵʽZn��Cu��2e-��Zn2+��Cu2+��2OH-��Cu��OH��2��Zn��OH��2��֪������Ϊ0.15mol��������ֻ��Cu��OH��2��������Ϊ0.15mol��98g/mol=14.7��������ֻ��Zn��OH��2��������Ϊ0.15mol��99g/mol=14.85��������B����һ�����ʣ�ȡ���ֹ���C���Թ��У��������������ᣬ�г�������ζ���������������Ϊ���⣬��ʵ��Ϊ�Ʊ�����п����BӦΪZnS������C�к���ZnS��CuS�������ᷴӦ�������������Ϊ��п����ӦΪ��ZnS+2H+=H2S��+Zn2+��
�ʴ�Ϊ��14.7��14.85g��ZnS+2H+=H2S��+Zn2+��
��5��Na2S����Һ��ˮ���Լ��ԣ������Ƶõ�ZnS�лẬ�н϶��Zn��OH��2��
�ʴ�Ϊ��Zn��OH��2��
���� ���⿼�������ú�ͭ�����Ĵ�п�Ʊ�����п�����̷�����������ѧ���ķ�����ʵ��ͼ��������Ŀ��飬�漰���Ļ���������ܽ�ƽ��Ӧ�á�������ԭ��֪ʶ����������ܽ�ƽ����йؼ����ǽⱾ��ؼ�����Ŀ�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ��1ml 1mol/L��NaOH����Һ�еμ�1��2��2mol/LMgCl2��Һ�а�ɫ�������ɣ��ٵμ�2��0.1mol/LFeCl3��Һ�����ֺ��ɫ��������֤��Mg��OH��2��Ksp����Fe��OH��3 | |
| B�� | ��pH=3��CH3COOH��Һ��pH=11��NaOH��Һ�������Ϻ����Һ������ | |
| C�� | ��0.1mol/L��CH3COOH��Һ��0.1mol/L��NaOH��Һ�������Ϻ����Һ������ | |
| D�� | ij���ʵ���Һ����ˮ�������c��H+��=1��10-a mol/L����a��7�������Һ��pHһ��Ϊ14-a |
ʵ�����2֧�Թ��зֱ����5mL��Ũ�ȵ�H2C2O4��Һ��������һ֧�Թ����ȼ�������MnSO4�����ٸ�����5��0.1mol•L-1KMnO4��Һ����¼��Һ��ɫʱ�䣬���1��
| �� �� | δ��MnSO4���Թ� | ����MnSO4���Թ� |
| ��ɫʱ�� | 30s | 2s |
ʵ�����ȡ2֧�Թֱܷ����5mL��Ũ�ȵ�H2C2O4��Һ��������һ֧�Թ����ȼ���10��ϡ���ᣬ�ٸ�����5��0.1mol•L-1 KMnO4��Һ����¼��Һ��ɫʱ�䣬���2��
| �� �� | δ�μ�ϡ������Թ� | �μ���ϡ������Թ� |
| ��ɫʱ�� | 100s | 90s |
ʵ�����ȡ3֧�Թֱܷ����5mL��Ũ��H2C2O4��Һ��Ȼ�����Թ����ȷֱ����10�Ρ�1mL��2mLϡ������Һ���ٸ�����5��0.1mol•L-1KMnO4��Һ��Ȼ�������¶�Ϊ65���ˮԡ�м��ȣ���¼��Һ��ɫʱ�䣬���3��
| �� �� | ����10��ϡ������Թ� | ����1mLϡ������Թ� | ����2mLϡ������Թ� |
| ��ɫʱ�� | 70s | 100s | 120s |
��1��ʵ���ó��Ľ�����Mn2+���������̣��ڷ�Ӧ�����������ã��ӿ��˷�Ӧ���ʣ�
��2���Ƚ�ʵ���ó��Ľ�����ȷ���ǣ��٢ڢۣ�
���¶ȶԸ÷�Ӧ������Ӱ��
������Բ����KMnO4��Һ�ķ�Ӧ��Ӱ��
�ۼ����������ᣬ�ɴٽ������KMnO4��Һ��Ӧ��������������ᣬ��Ӧ���ʱȽ�С
�������Է�Χ�ڣ�pHֵԽС�Է�ӦԽ����
��3��д��������Һ������KMnO4��Һ��Ӧ�����ӷ���ʽ��5H2C2O4+2MnO4-+6H+=2Mn2++10CO2��+8H2O��ʵ�������ø÷�Ӧ�궨δ֪Ũ��H2C2O4��Һ���ζ��յ�������ǣ���Һ��ɫ����ɫ��Ϊdz�Ϻ�ɫ����30s���ı䣮�ζ���ɺ����Ӷ�ȡKMnO4��Һ����ᵼ�²��H2C2O4��Һ��Ũ��ƫ��ѡ�ƫ��ƫС����Ӱ�죩��
��4����ͬѧ���ݲ��ĵ��������KMnO4��Һ����H2C2O4�ķ�Ӧ����Ϊ��

����ʵ���У�ʵ����֤����������ǿ��ŵģ�
| A�� | pH=3��HNO3��pH=11��KOH��Һ | |
| B�� | pH=3��HNO3��pH=11�İ�ˮ | |
| C�� | pH=3��H2SO4��pH=11��NaOH | |
| D�� | pH=3��CH3COOH ��pH=11��Ba��OH��2��Һ |
 ��a��CH 3������ΪOH��������ʷ�����OH�ĸ�������Ϊ��������
��a��CH 3������ΪOH��������ʷ�����OH�ĸ�������Ϊ��������| A�� | m-a | B�� | n+m+a | C�� | m+1-a | D�� | m+2-a |
 �ڲ�ͬ���������ٿ��ܷ��������л���Ӧ����ȡ�� �ڼӳ� ����ȥ ������ �����������и�����ӽṹ��-OH�йصĿ��ܷ�ӦΪ��������
�ڲ�ͬ���������ٿ��ܷ��������л���Ӧ����ȡ�� �ڼӳ� ����ȥ ������ �����������и�����ӽṹ��-OH�йصĿ��ܷ�ӦΪ��������| A�� | �٢ڢܢ� | B�� | �٢ڢۢ� | C�� | �ڢۢܢ� | D�� | �٢ۢܢ� |
| ʵ�鲽�� | ��3-4mL 1mol/L AgNO3��Һ�е���1mol/L��Na2SO4��Һ����Ӧ��ȫ�� |
| ʵ������ | �ٲ�����ɫ���� |
| ���ӷ���ʽ | 2Ag++SO${\;}_{4}^{2-}$=Ag2SO4 |
| С�����۽��� | ȡ������Ӧ�����Һ���Թ��е�������1mol/L��NaCl ��Һ�����ڻ���ֵ���������˼��� |
| ������� | �����Ӧû���� ����������� |
| ֤������� | ����ۣ�������ɫ���������ɢܷ�Ӧ�����ȣ���Һ�����н϶�Ag+ |
| ���ӷ���ʽ | ��Ag++Cl-=AgCl�� |
| ���� | ����Cl-�����˴����İ�ɫ������˵��Ag++Cl- AgCl����Ӧ�ǿ��淴Ӧ�����ڷ�Ӧ�ȣ�����Ag+���ܷ�Ӧ�꣬�ų����������� AgCl����Ӧ�ǿ��淴Ӧ�����ڷ�Ӧ�ȣ�����Ag+���ܷ�Ӧ�꣬�ų����������� |
 ��
��