��Ŀ����
17��ij���ʳ�������ˮ�еĵ�Ԫ�ض���NH${\;}_{4}^{+}$��NH3•H2O����ʽ���ڣ��÷�ˮ�Ĵ�������������ʾ��
���̢�NaOH��Һ������pH��9��������30�棬ͨ���������ϳ������հ�
���̢����������õ������£�NH4+����������Ӧ��������NO3-��������Ӧ�������仯ʾ��ͼ��ͼ��

��1����һ����Ӧ�Ƿ��ȷ�Ӧ��ѡ����ȡ������ȡ������ж������Ƿ�Ӧ����������������������������
��2��1mol NH4+��aq��ȫ��������NO3-��aq�����Ȼ�ѧ����ʽ��NH4+��aq��+2O2��g���T2H+��aq��+H2O��l��+NO3-��aq������H=-346 kJ/mol��
���� ��1������Ӧ������������������������������Ӧ�Ƿ��ȵģ�
��2�����ͼ����ݸ�˹���������㷴Ӧ���ʱ䣮
��� �⣺��1����ͼ��֪���ʱ�С��0������Ӧ�������������������������������Է�ӦΪ���ȷ�Ӧ��
�ʴ�Ϊ�����ȣ���Ӧ����������������������������
��2����һ�����Ȼ�ѧ����ʽΪNH4+��aq��+1.5O2��g���TNO2-��aq��+2H+��aq��+H2O��l������H=-273KJ/mol���ڶ������Ȼ�ѧ����ʽΪ��NO2-��aq��+0.5O2��g���TNO3-��aq������H=-73KJ/mol�����ݸ�˹������NH4+��aq��+2O2��g���T2H+��aq��+H2O��l��+NO3-��aq������H=-346 kJ/mol��
�ʴ�Ϊ��NH4+��aq��+2O2��g���T2H+��aq��+H2O��l��+NO3-��aq������H=-346 kJ/mol��
���� ���⿼���˻�ѧ��Ӧ�������仯����Ŀ�ѶȲ���ע�����ͼ���з�Ӧ�������������������������Ĺ�ϵ�Լ���˹���ɵ�Ӧ�ã�

��ϰ��ϵ�д�
�����Ŀ
12������ʵ�����������ͽ��۾���ȷ���ǣ�������
| ѡ�� | ʵ����� | ���� | ���� |
| A | ��ˮ����ͨ�����ȵ����� | ��ĩ��� | ����ˮ�ڸ����·�Ӧ |
| B | ��KI��FeCl3��Һ���Թ��л�Ϻ���CCl4�������� | �²���Һ���Ϻ�ɫ | �����ԣ�Fe3+��I2 |
| C | ���ɵ��߶ȵ�ͭ˿���뵽ϡHNO3�� | ��Һ���� | Cu��ϡHNO3�����û���Ӧ |
| D | ��AgNO3��Һ�еμӹ�����ˮ | ��Һ���� | Ag+��NH3?H2O�ܴ������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
9�������������ʵıȽϣ���ȷ���ǣ�������
| A�� | ���ȶ��ԣ�Na2CO3��NaHCO3��H2CO3 | B�� | �۵㣺K��Na��Li | ||
| C�� | �ܽ�ȣ�NaHCO3��Na2CO3 | D�� | �����ԣ�Li+��Na+��K+ |
7�����a��ij�����к��еķ�����Ϊb����c�˸���������ʵ����ǣ�NA��ʾ�����ӵ���������������
| A�� | $\frac{bc}{a{N}_{A}}$ | B�� | $\frac{ac}{b{N}_{A}}$ | C�� | $\frac{ab}{c{N}_{A}}$ | D�� | $\frac{b}{ac{N}_{A}}$ |
 ʵ���ǽ��л�ѧ�о�����Ҫ�ֶ�֮һ����ش��������⣺
ʵ���ǽ��л�ѧ�о�����Ҫ�ֶ�֮һ����ش��������⣺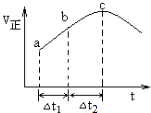

 ��
��
 ��
�� +O2$��_{��}^{����}$2
+O2$��_{��}^{����}$2 +2H2O��
+2H2O�� ��
��