��Ŀ����
6��������X��һ�����ϣ��ɲ�����ϩ��ױ�Ϊ��Ҫԭ�ϣ�������·�ߺϳɣ�
��֪��RCHO+CH3COOR��$\stackrel{CH_{3}CH_{2}ONa}{��}$RCH=CHCOOR��
��ش�
��1��CH2=CH2 ����6��ԭ�ӹ�ƽ�棻�ױ������ȱ���������ȡ����Ӧ���ҳ��õ���Ԫȡ�������ԭ���Dz�������������Ӱ���˱�����ʹ�����ϵ��������ڡ���λ��������ö���ȡ����
��2����Ӧ�ٵķ�Ӧ�����ǹ��գ�C��D�ķ�Ӧ������ȡ����Ӧ��
��3��B+D��F�Ļ�ѧ����ʽ
 ��
����4��E�й����ŵ�������ȩ����D��E�Ļ�ѧ����ʽ��2
 +O2$��_{��}^{����}$2
+O2$��_{��}^{����}$2 +2H2O��
+2H2O����5��X�Ľṹ��ʽ
 ��
����6�����ڻ�����X������˵����ȷ����AC��
A���ܷ���ˮ�ⷴӦ B������Ũ���ᷢ��ȡ����Ӧ
C����ʹBr2/CCl4��Һ��ɫ D���ܷ���������Ӧ
��7�����л�����������F��ͬ���칹�����BC��

���� ��ϩ��ˮ�ڴ��������·����ӳɷ�Ӧ����A��CH3CH2OH���Ҵ���������������B��CH3COOH���ױ��ڹ�����������������������ȡ����Ӧ�õ�CΪ ��C���������Ƶ�ˮ��Һ�з���ˮ�ⷴӦ��Ӧ�õ�DΪ
��C���������Ƶ�ˮ��Һ�з���ˮ�ⷴӦ��Ӧ�õ�DΪ ��D�����ᷢ��������Ӧ�õ�FΪ
��D�����ᷢ��������Ӧ�õ�FΪ ��D�������õ�EΪ
��D�������õ�EΪ �������Ϣ��֪XΪ
�������Ϣ��֪XΪ ��
��
��� �⣺��ϩ��ˮ�ڴ��������·����ӳɷ�Ӧ����A��CH3CH2OH���Ҵ���������������B��CH3COOH���ױ��ڹ�����������������������ȡ����Ӧ�õ�CΪ ��C���������Ƶ�ˮ��Һ�з���ˮ�ⷴӦ��Ӧ�õ�DΪ
��C���������Ƶ�ˮ��Һ�з���ˮ�ⷴӦ��Ӧ�õ�DΪ ��D�����ᷢ��������Ӧ�õ�FΪ
��D�����ᷢ��������Ӧ�õ�FΪ ��D�������õ�EΪ
��D�������õ�EΪ �������Ϣ��֪XΪ
�������Ϣ��֪XΪ ��
��
��1��CH2=CH2 Ϊƽ��ṹ������ԭ�Ӿ�����ͬһƽ�棬����6��ԭ�ӹ�ƽ�棻�ױ������ȱ���������ȡ����Ӧ���ҳ��õ���Ԫȡ�������ԭ���ǣ���������������Ӱ���˱�����ʹ�����ϵ��������ڡ���λ��������ö���ȡ����
�ʴ�Ϊ��6����������������Ӱ���˱�����ʹ�����ϵ��������ڡ���λ��������ö���ȡ����
��2����Ӧ�ٵķ�Ӧ�����ǹ��գ�C��D�ķ�Ӧ������ȡ����Ӧ��
�ʴ�Ϊ�����գ�ȡ����Ӧ��
��3��B+D��F�Ļ�ѧ����ʽ�� ��
��
�ʴ�Ϊ�� ��
��
��4��EΪ �������ŵ�������ȩ����D��E�Ļ�ѧ����ʽ�ǣ�2
�������ŵ�������ȩ����D��E�Ļ�ѧ����ʽ�ǣ�2 +O2$��_{��}^{����}$2
+O2$��_{��}^{����}$2 +2H2O��
+2H2O��
�ʴ�Ϊ��ȩ����2 +O2$��_{��}^{����}$2
+O2$��_{��}^{����}$2 +2H2O��
+2H2O��
��5��X�Ľṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��6��������XΪ ��
��
A��X�����������ܷ���ˮ�ⷴӦ����A��ȷ��
B�����б�������Ũ���ᷢ��ȡ����Ӧ����B����
C������̼̼˫������ʹBr2/CCl4��Һ��ɫ����C��ȷ��
D������ȩ�������ܷ���������Ӧ����D����
��ѡ��AC��
��7����������BC�ķ���ʽ��F�� ����ͬ���ṹ��ͬ����FΪͬ���칹�壬
����ͬ���ṹ��ͬ����FΪͬ���칹�壬
�ʴ�Ϊ��BC��
���� ���⿼���л�����ƶ���ϳɣ���������л���ķ���ʽ��ṹ�����ƶϣ���Ҫѧ���������չ����ŵ�������ת�����Ѷ��еȣ�

| A�� | 34 | B�� | 8.5 | C�� | 17 | D�� | 16 |
| A�� | Al2��SO4��3 | B�� | Na2CO3 | C�� | AlCl3 | D�� | KNO3 |


 A��һ�ֺ���ɫ���������B��D�ǽ������ʣ�J��һ��������ˮ�İ�ɫ��������Ⱥ��������ֽ⣮
A��һ�ֺ���ɫ���������B��D�ǽ������ʣ�J��һ��������ˮ�İ�ɫ��������Ⱥ��������ֽ⣮
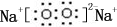 ��B�dz�����Һ̬�������ϡ��Һ�ױ����ֽ⣬��ʹ�õĴ���ΪAB��������ţ�
��B�dz�����Һ̬�������ϡ��Һ�ױ����ֽ⣬��ʹ�õĴ���ΪAB��������ţ�