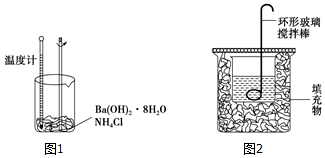
��Ŀ����
19��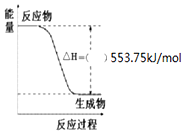 ��Ҫ��д��298K��101kPaʱ���з�Ӧ���Ȼ�ѧ����ʽ��
��Ҫ��д��298K��101kPaʱ���з�Ӧ���Ȼ�ѧ����ʽ����1��3mol NO2��g����1mol H2O��1����Ӧ����HNO3��aq����NO��g��������138kJ��3NO2��g��+H2O��l��=2HNO3��aq��+NO��g����H=-138 kJ/mol��
��2����ͼ��һ����������ȫȼ������CO2��1mol H2O��l���������仯ͼ��
������ͼ�е����������롰+����-����
��д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��C3H8��g��+5O2��g��=3CO2��g��+4H2O��l����H=-2215 kJ/mol��
��3��6g ̿���������в���ȫȼ������һ����̼���ų�55.2kJ���������Ȼ�ѧ����ʽΪC��s��+$\frac{1}{2}$O2 ��g��=CO��g����H=-110.4 kJ•mol-1��
���� ��1��3molNO2��Ӧ�ų�138kJ���������H=-138 kJ/mol��ע���ʱ�����ʵ����Ķ�Ӧ��ϵ��
��2���ٷ�Ӧ�������������������������˷�Ӧ�Ƿ��ȷ�Ӧ����Ӧ�������������������������÷�Ӧ�����ȷ�Ӧ��
��ȼ���ȵ���ָ��ȫȼ��1mol���������ȶ��������ų��������������Ȼ�ѧ����ʽ����д�������ش�
��3��������д�Ȼ�ѧ����ʽ�ķ�����������
��� �⣺��1��3molNO2��Ӧ�ų�138kJ��������H=-138 kJ/mol�����Ȼ�ѧ����ʽΪ3NO2��g��+H2O��l��=2HNO3��aq��+NO��g����H=-138 kJ/mol��
�ʴ�Ϊ��3NO2��g��+H2O��l��=2HNO3��aq��+NO��g����H=-138 kJ/mol��
��2���ٸ���ͼ����Ϣ����Ӧ�������������������������˷�Ӧ�Ƿ��ȷ�Ӧ�������ʱ��Ǹ�ֵ���ʴ�Ϊ��-��
��ȼ���ȵ���ָ��ȫȼ��1mol���������ȶ����������̼�����Һ̬ˮ���ų���������ͼ����һ����������ȫȼ������CO2��1mol H2O��l�������������Ա�����ȫȼ������CO2��4mol H2O��l��������Ϊ��553.75kJ/mol��4=2215.0 kJ/mol������C3H8��g��+5O2��g���T3CO2��g��+4H2O��l����H=-2215.0 kJ/mol��
�ʴ�Ϊ��C3H8��g��+5O2��g���T3CO2��g��+4H2O��l����H=-2215.0 kJ/mol��
��3��6g ̿���������в���ȫȼ������һ����̼���ų�55.2kJ��������1mol̿���������в���ȫȼ������һ����̼���ų�110.4kJ�������Ȼ�ѧ����ʽΪ��C��s��+$\frac{1}{2}$O2 ��g��=CO��g����H=-110.4 kJ•mol-1���ʴ�Ϊ��C��s��+$\frac{1}{2}$O2 ��g��=CO��g����H=-110.4 kJ•mol-1��
���� ���⿼��ѧ����Ӧ�������ȵ��ж��Լ��Ȼ�ѧ����ʽ����д��֪ʶ�������ۺ�֪ʶ�Ŀ��飬�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д� ��2L�ܱ������ڣ�800��ʱ��Ӧ��2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
��2L�ܱ������ڣ�800��ʱ��Ӧ��2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����| ʱ�䣨s�� | 0 | 1 | 2 | 3 | 4 | 5 |
| n��NO����mol�� | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
��֪��K300��C��K350��C����÷�Ӧ�Ƿ��ȷ�Ӧ��
��2��ͼ�б�ʾNO2�ı仯��������b����O2��ʾ��0��2s�ڸ÷�Ӧ��ƽ������v=1.5��10-3mol•L-1•s-1��
��3����˵���÷�Ӧ�Ѵﵽƽ��״̬����bc��
a��v ��NO2��=2v ��O2�� b��������ѹǿ���ֲ���
c��v����NO��=2v�� ��O2�� d���������ܶȱ��ֲ���
��4��Ϊʹ�÷�Ӧ�ķ�Ӧ����������ƽ��������Ӧ�����ƶ�����c��
a����ʱ�����NO2���� b���ʵ������¶�
c������O2��Ũ�� d��ѡ���Ч����
HNO2��һ�����ᣬ��������ĵ���ƽ�ⳣ�����±���
| ���� | HNO2 | HClO | H2CO3 | H2SO3 |
| ����ƽ�ⳣ�� ��25�棩 | Ki=5.1��10-4 | Ki=2.98��10-8 | $\begin{array}{l}{K_{i1}}=4.3��{10^{-7}}\\{K_{i2}}=5.6��{10^{-11}}\end{array}$ | $\begin{array}{l}{K_{i1}}=1.54��{10^{-2}}\\{K_{i2}}=1.02��{10^{-7}}\end{array}$ |
��6���������ӷ���ʽ��ȷ����BD
A��2ClO-+H2O+CO2��2HClO+CO32-
B��2HNO2+CO32-��2NO2-+H2O+CO2��
C��H2SO3+CO32-��CO2+H2O+SO32-
D��Cl2+H2O+2CO32-��2HCO3-+Cl-+ClO-
��7�������£�pH=3��HNO2��Һ��pH=11��NaOH��Һ�������Ϻ���Һ������Ũ���ɴ�С��˳��Ϊc��NO2-����c��Na+����c��H+����c��OH-����
��Fe��s��+H2O��g��?FeO��s��+H2��g��?K2
��H2��g��+CO2��g��?H2O��g��+CO��g��?K3
����֪�ڲ�ͬ���¶��£�K1��K2��ֵ�����
| �¶ȡ� | K1 | K2 |
| 500 | 1.00 | 3.15 |
| 700 | 1.47 | 2.26 |
| 900 | 2.40 | 1.60 |
��2����Ӧ�ڵ�ƽ�ⳣ������ʽΪK=$\frac{[H{\;}_{2}]}{[H{\;}_{2}O]}$���˷�Ӧ�ں��º��ݵ��ܱ�װ���н��У��ܳ��˵���˷�Ӧ�Ѵﵽƽ��ı�־��AB��
A�������ƽ����Է����������ٸı� B����������������ٸı�
C��������ܷ��������ٸı� D��������ѹǿ����ʱ��仯���仯
��3����900��ʱ���з�Ӧ �ۣ���ƽ�ⳣ��K3Ϊ1.5���������ֵ�����ʱ��H��0�������������������=����������֪�÷�Ӧ���ڸ���ʱ�Է����У����S��0�������������������=������
��4����500��ʱ���з�Ӧ �ۣ���ijʱ��ʱC��H2��=C��CO2��=C��H2O��=C��CO�������ʱ����ǡ��������ﵽƽ��״̬����û�дﵽƽ��״̬�����ʱ�÷�Ӧ�������棨����������桱��������У�����Ϊ��Ũ����=$\frac{[CO]•[H{\;}_{2}O]}{[H{\;}_{2}]•[CO{\;}_{2}]}$=1��K3�����Դ�ʱƽ�����淴Ӧ�����ƶ���
| A�� | ������ȡ����ʱ��Ӧѡ���л���ȡ��������ȡ�����ܶȱ������ˮ | |
| B�� | H2��ԭCuOʱ����ͨH2�����CuO����Ӧ��Ϻ���ֹͣͨH2�ƾ��� | |
| C�� | �����������ʱ���������е�����ˮӦ���¿ڽ����Ͽڳ� | |
| D�� | ��ҹ������ú��й©ʱ���ȴƣ���Ѹ�ٿ���ͨ�� |
 ij100mL��Һ�к��еIJ�������Ũ�ȴ�С��ͼ��ʾ������Һ���ܻ�����Fe3+��Ba2+��K+��OH-��NO3-��CO32-��SO42-��Ϊ�˽�һ��ȷ�ϣ��Ը���Һ����ʵ���⣺
ij100mL��Һ�к��еIJ�������Ũ�ȴ�С��ͼ��ʾ������Һ���ܻ�����Fe3+��Ba2+��K+��OH-��NO3-��CO32-��SO42-��Ϊ�˽�һ��ȷ�ϣ��Ը���Һ����ʵ���⣺ ��ijѧ����0.2000mol•L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷ�Ϊ���¼�����
��ijѧ����0.2000mol•L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷ�Ϊ���¼�����