��Ŀ����
8����ѧ��Ӧ������һ�������������仯��ijͬѧͨ������ʵ�����̽������̽����ѧ��Ӧ�е������仯��ͨ����ͼ1ʵ�������ձ��е��¶Ƚ��ͣ�
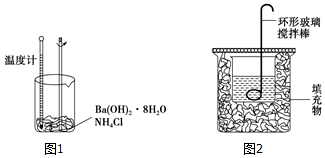
��1��Ba��OH��2•8H2O��NH4Cl��Ӧ�Ļ�ѧ����ʽ��Ba��OH��2•8H2O+2NH4Cl=BaCl2+8H2O+2NH3•H2O��
��2���ӷ�Ӧ�ȵĽǶȷ������÷�Ӧ�������ȣ�����ȡ����ȡ�����Ӧ����������ԭ�Ƕȷ������÷�Ӧ���ڷ�������ԭ���������ԭ����������ԭ������Ӧ���ӻ�����Ӧ�����Ϸ������÷�Ӧ���ڸ��ֽⷴӦ��
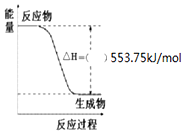
�ⶨϡ���������������Һ��Ӧ���к��ȣ�ʵ��װ����ͼ2��ʾ����
��1��ͼ����ȱ�ٵ�һ���������¶ȼƣ�
��2��ʵ��ʱ���β�����������˶�������a��
a�������˶�������������b�������˶�
c��˳ʱ���˶� d����ʱ���˶�
��3��д����Ӧ���Ȼ�ѧ����ʽΪHCl��aq��+NaOH��aq��=NaCl��aq��+H2O��l����H=-57.3KJ/mol���к���Ϊ57.3kJ•mol-1����
��4����ͬѧÿ�ηֱ�ȡ0.50mol•L-1 50mL NaOH��Һ��0.50mol•L-130mL�������ʵ�飬ͨ�����ʵ��ⶨ�к��ȡ�H=-53.5kJ•mol-1����57.3kJ•mol-1��ƫ�����ƫ���ԭ�������acd������ĸ����
a��ʵ��װ�ñ��¡�����Ч����
b������Ͳ��ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳⶨ������Һ���¶ȣ�
���� I����1���������ֽⷴӦ�����Ȼ�����һˮ�ϰ���ˮ��
��2���÷�Ӧ������������Ԫ�صĻ��ϼ۱仯��Ϊ�������뻯���ﷴӦ�����µĻ����
��1��ͼ2��ȱ���¶ȼƲⶨ�¶ȣ�
��2�����β�������������˶����裻
��3��ϡ����Ӧ����1molˮ������Ϊ�к��ȣ�
��4��a��װ�ñ��¡�����Ч�����õ�����ƫС��
b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ�
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�����ɢʧ�϶ࣻ
d���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ��������ʼ�¶�ƫ�ߣ�
��� �⣺��1��Ba��OH��2•8H2O��NH4Cl��Ӧ�Ļ�ѧ����ʽΪBa��OH��2•8H2O+2NH4Cl=BaCl2+8H2O+2NH3•H2O��
�ʴ�Ϊ��Ba��OH��2•8H2O+2NH4Cl=BaCl2+8H2O+2NH3•H2O��
��2���÷�Ӧ����������Ϊ���ȷ�Ӧ����Ԫ�صĻ��ϼ۱仯�����ڷ�������ԭ��Ӧ��Ϊ�������뻯���ﷴӦ�����µĻ�������ڸ��ֽⷴӦ��
�ʴ�Ϊ�����ȣ���������ԭ�����ֽⷴӦ��
��1��ͼ����ȱ�ٵ�һ���������¶ȼƣ��ʴ�Ϊ���¶ȼƣ�
��2�����β�������������˶����裬�ʴ�Ϊ��a��
��3����Ӧ���Ȼ�ѧ����ʽΪΪHCl ��aq��+NaOH��aq��=NaCl��aq��+H2O��l����H=-57.3KJ/mol��
�ʴ�Ϊ��HCl ��aq��+NaOH��aq��=NaCl��aq��+H2O��l����H=-57.3KJ/mol��
��4��a��װ�ñ��¡�����Ч�����õ�����ƫС���к��ȵ���ֵƫС����a��ȷ��
b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ��к��ȵ���ֵƫ��b����
c���ֶ�ΰ�NaOH ��Һ����ʢ�������С�ձ��У�����ɢʧ�϶࣬����¶�ƫ�ͣ��к��ȵ���ֵƫС����c��ȷ��
d���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ��������ʼ�¶�ƫ�ߣ��¶Ȳ�ƫС����õ�����ƫС���к��ȵ���ֵƫС����d��ȷ��
�ʴ�Ϊ��acd��
���� ���⿼���к��Ȳⶨʵ�飬Ϊ��Ƶ���㣬���ղⶨԭ����ʵ�������ʵ�鼼��Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע���к��ȵ���ֵ������������Ŀ�ѶȲ���

| A�� | BrCl�Ļ�ѧ���ʺ�Cl2���ƣ�Cl2��ˮ��Ӧ��������ԭ��Ӧ��BrCl+H2O=HCl+HBrOҲ��������ԭ��Ӧ | |
| B�� | �����������£�������������������ͭ��Ӧ����������Ҳ����������ͭ��Ӧ | |
| C�� | ������ʹ���Ը��������ɫ���ױ�Ҳ����ʹ���Ը��������ɫ | |
| D�� | CO2��SiO2��Ϊ���������CO2����NaOH��Һ��Ӧ��SiO2Ҳ����NaOH��Һ��Ӧ |
| A�� | SO2 | B�� | NO2 | C�� | PM2.5 | D�� | CO2 |
 ��Ҫ��д��298K��101kPaʱ���з�Ӧ���Ȼ�ѧ����ʽ��
��Ҫ��д��298K��101kPaʱ���з�Ӧ���Ȼ�ѧ����ʽ��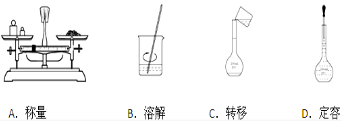

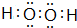 ��
�� ������������ͼ��
������������ͼ��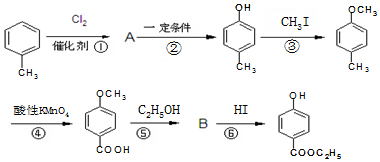
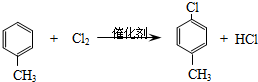 ��
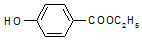
�� ��-X��-YΪȡ�������Ƕ��ǻ�������������ͬ���칹�����ܷ���������Ӧ����-X�Ľṹ��ʽ������-OH��-CH2OH ��-OCH3��
��-X��-YΪȡ�������Ƕ��ǻ�������������ͬ���칹�����ܷ���������Ӧ����-X�Ľṹ��ʽ������-OH��-CH2OH ��-OCH3��