��Ŀ����
6�� ij100mL��Һ�к��еIJ�������Ũ�ȴ�С��ͼ��ʾ������Һ���ܻ�����Fe3+��Ba2+��K+��OH-��NO3-��CO32-��SO42-��Ϊ�˽�һ��ȷ�ϣ��Ը���Һ����ʵ���⣺
ij100mL��Һ�к��еIJ�������Ũ�ȴ�С��ͼ��ʾ������Һ���ܻ�����Fe3+��Ba2+��K+��OH-��NO3-��CO32-��SO42-��Ϊ�˽�һ��ȷ�ϣ��Ը���Һ����ʵ���⣺����ϸ�۲죬����Һ����ɫ��������һ״̬��
����100mL��Һ�е���ϡ���ᣬ��23.3g��ɫ�����������ټ�ϡ���ᣬ��ɫ��������ʧ���ش��������⣺�ɴ˿�֪�����˺���Na+��Mg2+��Cl-�⣬����Һ�п϶������е����Ӽ������ʵ���Ũ��ΪBa2+��Ũ��Ϊ1mol/L����NO3-��Ũ�ȡ�1mol/L�����϶�û�е�������Fe3+��OH-��CO32-��SO42-�����ܺ��е�������K+��
���� �ɢ���Һ����ɫ��������һ״̬����֪һ������Fe3+����Ba2+������CO32-��SO42-���棻
�ɢ���100mL��Һ�е���ϡ���ᣬ��23.3g��ɫ�����������ټ�ϡ���ᣬ��ɫ��������ʧ����֪��ɫ����Ϊ���ᱵ����һ����Ba2+������CO32-��SO42-����Һ������Mg2+����OH-���Դ������
��� �⣺�ɢ���Һ����ɫ��������һ״̬����֪һ������Fe3+����Ba2+������CO32-��SO42-���棻
�ɢ���100mL��Һ�е���ϡ���ᣬ��23.3g��ɫ�����������ټ�ϡ���ᣬ��ɫ��������ʧ����֪��ɫ����Ϊ���ᱵ����һ����Ba2+����Ũ��Ϊ$\frac{\frac{23.3g}{233g/mol}}{0.1L}$=1mol/L������CO32-��SO42-����Һ������Mg2+����OH-�����ͼ��֪Na+��Mg2+��Cl-��Ũ�ȷֱ�Ϊ1.0mol/L��0.5mol/L��3.0mol/L���ɵ���غ��֪��1mol/L��2+1.0mol/L+0.5mol/L��2��3.0mol/L����һ����������NO3-����Ũ�ȡ�1mol/L������ȷ���Ƿ�K+��
�ʴ�Ϊ��Ba2+��Ũ��Ϊ1mol/L����NO3-��Ũ�ȡ�1mol/L����Fe3+��OH-��CO32-��SO42-��K+��
���� ���⿼�����ӵ��ƶϣ�Ϊ��Ƶ���㣬��������֮��ķ�Ӧ������Ϊ���Ĺؼ������ط������ƶ������Ŀ��飬ע���ɫ�����ƶϼ�����غ�Ӧ�ã���Ŀ�ѶȲ���

| A�� |  | B�� |  | C�� |  | D�� |  |
| A�� | ��ȥNH4Cl��Һ�е�FeCl3��������Һ�м��백ˮ����pH | |
| B�� | ��ȥ��������Cu2+��Hg2+��������Һ�м���H2S��Na2S�ȳ����� | |
| C�� | ��ȥij��Һ�е�SO42-������Һ�м���þ�� | |
| D�� | ��ȥZnCl2��Һ�е�Fe3+������Һ�м���ZnO |

 ��
��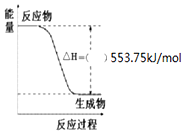 ��Ҫ��д��298K��101kPaʱ���з�Ӧ���Ȼ�ѧ����ʽ��
��Ҫ��д��298K��101kPaʱ���з�Ӧ���Ȼ�ѧ����ʽ��