��Ŀ����
15��ʵ��������ʹ��0.100mol/L��Na2CO3��Һ400mL��������Ӧ��Һ����ش��������⣺������ƽȷ��ȡNa2CO3����5.3g������С�ձ��У�����������ˮ�ܽ⣮
�ڽ������õ���Һת����500mL����ƿ�У�
��������������ˮϴ�Ӳ��������ձ��ڱ�2��3�Σ�����ϴ��ҺС�ĵ�ע������ƿ�У�������
�ܼ���������ƿ�л�����������ˮ��Һ�����̶���1��2cm�������ý�ͷ�ι���εμ�����ˮ����Һ�İ�Һ��������̶������У���������������δ������������ƣ�
�ݰ�����ƿ���Ǻã��������µߵ������ҡ�ȣ����������Һ����ڿ̶��ߣ����貹���ˮ�𣿲���Ҫ�����Ҫ������Ҫ����
�������������ڡ�A��ƫС��B��ƫ��C����Ӱ�족��ѡ������ʵ���ĸ��գ�
��1������ƿ��ʹ��֮ǰ�����������ˮ���С���©������������������ƿ��ʪ����������Һ��Ũ�Ȼ�C��
��2����Щ�����ܽ����ȣ���δ����ȴ��ת��������ƿ����������Һ��Ũ�Ȼ�B��
��3����δ��ϴ�ӻ�ϴ��Һ��ת��ʱ����������ʵ�����ϣ���������Һ��Ũ�Ȼ�A��
���� ������m=CVM������Ҫ���ʵ�������
������������Һ���������ƿ���ѡ������ƿ��
��Ϊ��֤����ȫ��ת�Ƶ�����ƿ��Ӧϴ��մ�����������ձ��ڱ��ϵ����ʣ�
�����ݶ��ݵ���ȷ������𣬷��Dz�������ʵ��ʧ���Ҳ��ܲ��ȵĶ���Ҫ����������Һ��
�ݶ���ҡ�Ⱥ���Һմ��ƿ���ϵ���Һ���½���
�������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$�����жϣ�
��� �⣺������0.100mol/L��Na2CO3��Һ400mL��Ӧѡ��500mL����ƿ��ʵ������500mL��Һ����Ҫ���ʵ�����m=0.100mol/L��0.5L��106g/mol=5.3g��
�ʴ�Ϊ��5.30��
������0.100mol/L��Na2CO3��Һ400mL��ʵ����û��400mL����ƿ������Ӧѡ��500mL����ƿ��
�ʴ�Ϊ��500��
��Ϊ��֤����ȫ��ת�Ƶ�����ƿ��Ӧϴ��մ�����������ձ��ڱ��ϵ����ʣ�
�ʴ�Ϊ�����������ձ��ڱڣ�
�ܼ���������ƿ�л�����������ˮ��Һ�����̶���1��2cm�������ý�ͷ�ι���εμ�����ˮ����Һ�İ�Һ��������̶������У�����������������ʵ��ʧ���������ȣ���Ҫ�������ƣ�
�ʴ�Ϊ����ͷ�ι� �������ƣ�
�ݶ���ҡ�Ⱥ���Һմ��ƿ���ϵ���Һ���½���������������������Ҫ�����ˮ��
�ʴ�Ϊ������Ҫ��
�ޣ�1������ƿ��ʹ��֮ǰ�����������ˮ���С���©������������������ƿ��ʪ������ʱ��Ȼ��Ҫ��������ˮ�����Զ����ʵ����ʵ�������Һ������������Ӱ�죬��ҺŨ�Ȳ��䣬��ѡ��C��
��2����Щ�����ܽ����ȣ���δ����ȴ��ת��������ƿ����ȴ����Һ���ƫС����������Һ��Ũ�Ȼ�ƫ�ߣ���ѡ��B��
��3����δ��ϴ�ӻ�ϴ��Һ��ת��ʱ����������ʵ�����ϣ����²���������ģ����ʵ����ʵ���ƫС����������Һ��Ũ�Ȼ�ƫ�ͣ���ѡ��A��
�ʴ�Ϊ����1��C ��2��B ��3��A��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ���Ͳ��������ǽ���ؼ���ע������ƿ����ѡ���ʹ�÷�����ע���������ķ�����

 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�| A�� | �Ƴ�������������Ӧ����Na2O�������¶����� Na2O�����ʼӿ� | |
| B�� | �����£�ͭ��ϡ���ᷴӦ����NO�����������Ũ�ȣ�����NO�����ʼӿ� | |
| C�� | п��ϡ�����Ʊ���������������������ͭ��Һ������H2�����ʼӿ� | |
| D�� | ���ܱ������з����ķ�Ӧ2NO2?2NO+O2 ��С���������ѹǿ��V��������С��V���棩���� |
| A�� | ��״���£�5.6L O2��������ʱת�Ƶ�����һ��ΪNA | |
| B�� | �ö��Ե缫���CuSO4��Һ���������0.1mol Cu��OH��2��ʹ��Һ��ԭ�����·��ת�Ƶ��ӵ���ĿΪ0.2NA | |
| C�� | ��֪3BrF3+5H2O�THBrO3+Br2+9HF+O2�������5molH2O�μ�������ԭ��Ӧ������ˮ��ԭ��BrF3������ĿΪ3NA | |
| D�� | 142gNa2SO4��Na2HPO4���������У�������������Ϊ3NA |
240mL 0.1mol•L-1�����ᣬ��ش��������⣺
��1��Ũ��������ʵ���Ũ��Ϊ12.5mol/L����
��2������240mL 0.1mol•L-1������
| Ӧ��ȡŨ�������/mL | Ӧѡ������ƿ�Ĺ��/mL |
A����30mLˮϴ��2��3�Σ�ϴ��Һ��ע������ƿ����
B����5mLȷ��ȡ�����Ũ�����������ز����������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע������ƿ��
D��������ƿ�ǽ�����ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ��Һ����ʹ�ǡ����̶�������
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1-2cm��
��4������A�У���ϴ��Һ����������ƿ����Ŀ����ϴ��Һ�к������������ʣ�Ϊ�������ʵ����ļ��٣�Ӧ��ϴ��Һȫ��ת�Ƶ�����ƿ��
��5����ʵ������г������������δ�����
�ټ�����ˮʱ���������˿̶��ߣ��������ƣ�
��������ƿ��ת����Һʱ������Һ�ν�������ƿ���棺�������ƣ�
��6����ʵ�����������������Һ��Ũ����ƫ�ߣ�ƫ�ͻ��Dz��䣿
A������ʱ���ӿ̶���ƫ�ͣ�
B�����ǽ�ϴ��Һ��������ƿƫ�ͣ�
C������ƿ�ڱڸ���ˮ���δ���ﴦ�����䣻
D���ܽ��û����ȴ����ж���ƫ�ߣ�
 4���������������Ԫ�ص����λ�������Ԫ��X�ĺ����������Ԫ��M��2����Y��������������ԣ��ش��������⣮
4���������������Ԫ�ص����λ�������Ԫ��X�ĺ����������Ԫ��M��2����Y��������������ԣ��ش��������⣮ ��������ӣ�MN��2�ĵ���ʽ��
��������ӣ�MN��2�ĵ���ʽ��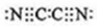 ��X�γɵ����ӵĵ���ʽ��Mg2+��
��X�γɵ����ӵĵ���ʽ��Mg2+�� ClO2��Cl2�������������������ˮ�������߱��ʵȷ���Ӧ�ù㷺��ij��ȤС��ͨ��ͼ1װ�ã��г�װ���ԣ������Ʊ������ա��ͷź�Ӧ�ý������о���
ClO2��Cl2�������������������ˮ�������߱��ʵȷ���Ӧ�ù㷺��ij��ȤС��ͨ��ͼ1װ�ã��г�װ���ԣ������Ʊ������ա��ͷź�Ӧ�ý������о���

 ��CH4��H2OΪԭ�ϣ�ͨ�����з�Ӧ���Ʊ��״���
��CH4��H2OΪԭ�ϣ�ͨ�����з�Ӧ���Ʊ��״���