��Ŀ����
7������ʵ���ܴﵽԤ��Ŀ���DZ�ţ�������| ʵ������ | ʵ��Ŀ�� | |
| A | ��1mL 1%��NaOH��Һ�м���2mL 2%��CuSO4��Һ�����ټ���0.5mL�л���X��������к�δ����ש��ɫ���� | ��֤X�ṹ�в�����ȩ�� |
| B | �ڻ����������ӵı��еμ�������ˮ���������� | ��ȥ���������ı��� |
| C | ��������NaOH��Һ���������ٷֲ㣬��ȴ���ϡ���������ԣ��ٵμ�AgNO3��Һ | ��֤±��ԭ��Ϊ��ԭ�� |
| D | ���Ҵ���Ũ���Ṳ���Ƶõ�����ͨ������KMnO4��Һ�� | ���������к�����ϩ |
| A�� | A | B�� | B | C�� | C | D�� | D |
���� A��������������ͭ����Һ����ȩ��ʱ���������ڼ��������£�
B�����Ӻ��巢��ȡ����Ӧ�����屽���屽���������ܻ��ܣ�
C��±����ˮ�������NaX������������������������Ӧ����AgX�����ó�������ɫ��ȡ��±��ԭ�ӣ�
D���Ҵ��ӷ����ӷ������Ҵ�Ҳ�ܹ�ʹ���Ը��������Һ��ɫ��
��� �⣺A������������ͭ����Һ����ȩ��ʱ���������ڼ��������£���ʵ����NaOH���㵼��NaOH������ͭ�����Һ���Ǽ��ԣ�����ʵ�鲻�ɹ�����A����
B�����Ӻ��巢��ȡ����Ӧ�����屽���屽���������ܻ��ܣ�Ӧ����NaOH�ͱ��ӷ�Ӧ���ɿ����Եı����ƣ�Ȼ���Һ��ȥ���еı��ӣ���B����
C��±�����м�������NaOHˮ��Һ�����ȣ�ˮ�������NaX������������������������Ӧ����AgX�����ó�������ɫ��ȡ��±��ԭ�ӣ���C��ȷ��
D���ӷ��������Ҵ��ܹ�ʹ���Ը��������Һ��ɫ����������ϩ�ļ��飬Ӧ������ˮ������ϩ����D����
��ѡC��
���� ���⿼�黯ѧʵ�鷽�������ۣ�Ϊ��Ƶ���㣬�漰�л�����Ʊ���ˮ�⡢��ȥʵ�鼰����ļ���ȣ������л�������ʼ�ʵ��ԭ��Ϊ���Ĺؼ���ע��ʵ��IJ����ԡ������Է�������Ŀ�ѶȲ���

| A�� | 15g����-CH3�������еĵ�������10 NA�� | |
| B�� | ��״���£�2.24 L CHCl3��ԭ������Ϊ0.5 NA�� | |
| C�� | 4.2g C3H6�к��е�̼̼˫����һ��Ϊ0.1NA | |
| D�� | �����£�14g��ϩ�ͱ�ϩ�Ļ��������ԭ����Ϊ3NA�� |
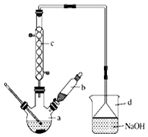 �屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ������װ��ʾ��ͼ���й����������
�屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ������װ��ʾ��ͼ���й����������| �� | �� | �屽 | |
| �ܶ�/g•cm-3 | 0.88 | 3.10 | 1.50 |
| �е�/�� | 80 | 59 | 156 |
| ˮ���ܽ�� | �� | �� | �� |
��1����a�м���15mL��ˮ����������м����b��С�ļ���4.0mLҺ̬�壮�ٽ�b�е�Һ����������a�У���ַ�Ӧ��װ��a����Ҫ��Ӧ��2Fe+3Br2�T2FeBr3��
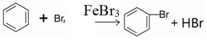 ��װ��d����������HBr������������ֹ������
��װ��d����������HBr������������ֹ��������2����Ӧ�����У�a����¶����ߣ�Ϊ���ԭ�ϵ������ʣ��ɲ�ȡ���´�ʩ��
�ٲ���װ��c����������������������Ҫ������C6H6��Br2��
�������¶ȼƿ����¶ȣ����˵��¶ȷ�ΧΪC������ţ���
A����156��B��59��-80��C����59��
��3��Һ�����������в�������ᴿ��
����a�м���10mLˮ��Ȼ����˳�ȥδ��Ӧ����м��
����Һ������10mLˮ��8mL 10%��NaOH��Һ��10mLˮϴ�ӣ�
����ֳ��Ĵ��屽�м�����������ˮ�Ȼ��ƣ����á����ˣ�
�������NaOH��Һϴ�ӵ���Ҫ�����ǣ��û�ѧ����ʽ��ʾ��Br2+2NaOH=NaBr+NaBrO+H2O��
��4�������Ϸ���������屽�л����е���Ҫ����Ϊ����Ҫ��һ���ᴿ�����в����б������C������ţ���
A����Һ B������ C������ D����ȡ
��5���ڸ�ʵ���У�a���ݻ����ʺϵ���B������ţ���
A.25mL B.50mL C.250mL D.500mL��
| A�� | S��g��+O2��g��=SO2��g����H3S��s��+O2��g��=SO2��g����H2�����H1����H2 | |
| B�� | Zn��s��+CuSO4��aq��=ZnSO4��aq��+Cu��s����H=-216kJ•mol-1����Ӧ�������������������� | |
| C�� | ��֪C��ʯī•s��=C�����ʯ•s����H��0����ʯī�Ƚ��ʯ�ȶ� | |
| D�� | ��ͬ�����£����1mol��ԭ�������е�����ΪE1•1mol����������е�����ΪE2����2E1=E2 |
| A�� | SO2��ת����Ϊ60% | |
| B�� | SO3�IJ���Ϊ60% | |
| C�� | ƽ��ʱ��ѹǿ����ʼѹǿ֮��Ϊ7��8 | |
| D�� | ƽ��ʱV��SO2����V��O2����V��SO3��=3��3��1 |
