��Ŀ����
12��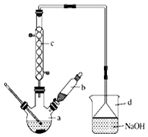 �屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ������װ��ʾ��ͼ���й����������
�屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ������װ��ʾ��ͼ���й����������| �� | �� | �屽 | |
| �ܶ�/g•cm-3 | 0.88 | 3.10 | 1.50 |
| �е�/�� | 80 | 59 | 156 |
| ˮ���ܽ�� | �� | �� | �� |
��1����a�м���15mL��ˮ����������м����b��С�ļ���4.0mLҺ̬�壮�ٽ�b�е�Һ����������a�У���ַ�Ӧ��װ��a����Ҫ��Ӧ��2Fe+3Br2�T2FeBr3��
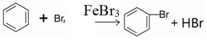 ��װ��d����������HBr������������ֹ������
��װ��d����������HBr������������ֹ��������2����Ӧ�����У�a����¶����ߣ�Ϊ���ԭ�ϵ������ʣ��ɲ�ȡ���´�ʩ��
�ٲ���װ��c����������������������Ҫ������C6H6��Br2��
�������¶ȼƿ����¶ȣ����˵��¶ȷ�ΧΪC������ţ���
A����156��B��59��-80��C����59��
��3��Һ�����������в�������ᴿ��
����a�м���10mLˮ��Ȼ����˳�ȥδ��Ӧ����м��
����Һ������10mLˮ��8mL 10%��NaOH��Һ��10mLˮϴ�ӣ�
����ֳ��Ĵ��屽�м�����������ˮ�Ȼ��ƣ����á����ˣ�
�������NaOH��Һϴ�ӵ���Ҫ�����ǣ��û�ѧ����ʽ��ʾ��Br2+2NaOH=NaBr+NaBrO+H2O��
��4�������Ϸ���������屽�л����е���Ҫ����Ϊ����Ҫ��һ���ᴿ�����в����б������C������ţ���
A����Һ B������ C������ D����ȡ
��5���ڸ�ʵ���У�a���ݻ����ʺϵ���B������ţ���
A.25mL B.50mL C.250mL D.500mL��
���� ��1��ʵ���Һϳ��屽�DZ���Һ���������������������·���ȡ����Ӧ�����屽���廯�⣻HBr��Һ���ӷ���������±����Ӧ�Ƿ��ȵģ�β������HBr���ӷ�����Br2��������������Һ���գ���ֹ��Ⱦ������
��2���ٱ���Һ���ڴ����������·�����Ӧ��ͬʱ���ܷ��ȣ�����Ӧ�ﱽ��Һ�嶼�ӷ���Ϊ���ٷ�Ӧ��ӷ������ԭ�ϵ������ʣ�������Һ������������
��Ϊ���ٷ�Ӧ��ӷ������ԭ�ϵ������ʿ���ͨ���¶ȵĿ�����ʵ�֣�ֻҪ�¶ȵ��ڶ��ߵķе�Ϳ����ˣ�
��3���屽�к����壬��NaOH��Һ����δ��Ӧ��Br2��Ӧϴ��ˮ�У�
��4���ɷ��������֪��������Ĵ��屽�к���δ��Ӧ�ı������뻥�ܵ�Һ�壬���ݷе㲻ͬ����������ķ������з��룻
��5��������ȡ�屽���ӵ�Һ�������Լ���Һ�����һ�㲻��������$\frac{2}{3}$��������$\frac{1}{3}$�����
��� �⣺��1��ʵ���Һϳ��屽�DZ���Һ���������������������·���ȡ����Ӧ�����屽���廯�⣬��Ӧ����ʽΪ2Fe+3Br2�T2FeBr3��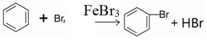 ��HBr��Һ���ӷ���������±����Ӧ�Ƿ��ȵģ�β������HBr���ӷ�����Br2��������������Һ���գ���ֹ��Ⱦ����������©�����ܷ�ֹ������
��HBr��Һ���ӷ���������±����Ӧ�Ƿ��ȵģ�β������HBr���ӷ�����Br2��������������Һ���գ���ֹ��Ⱦ����������©�����ܷ�ֹ������
�ʴ𰸣���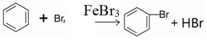 ������HBr������������ֹ������
������HBr������������ֹ������
��2���ٱ���Һ���ڴ����������·�����Ӧ��ͬʱ���ܷ��ȣ�����Ӧ�ﱽ��Һ�嶼�ӷ���Ϊ���ٷ�Ӧ��ӷ������ԭ�ϵ������ʣ�������Һ������������
�ʴ�Ϊ��C6H6��Br2��
�ڸ��ݱ��б���Һ��ķе㣬ѡ������¶ȣ�ȷ���¶��ڶ��ַ�Ӧ��ķе�֮�£���Ӧѡ���¶�Ϊ����59�棻
�ʴ�Ϊ��C��
��3���屽�к����壬��NaOH��Һ����δ��Ӧ��Br2���NaBr��NaBrOϴ��ˮ�У�����Ϊ��Br2+2NaOH=NaBr+NaBrO+H2O��
�ʴ�Ϊ��Br2+2NaOH=NaBr+NaBrO+H2O��
��4����Ӧ��õ����屽����������δ��Ӧ�ı��������屽���ܣ������ķе�ͣ����Բ�������ķ������з��룬�屽����ĸҺ�У�
�ʴ�Ϊ������C��
��5�����������У�����a�м���15mL��ˮ������b��С�ļ���4.0mLҺ̬�壬�����a�м���10mLˮ����Լ30mL������a���ݻ����ʺϵ���50mL��
�ʴ�Ϊ��B��
���� ���⿼�������Ʊ�ʵ�鷽������ƣ�Ϊ��Ƶ���㣬��Ŀ�Ѷ��еȣ���ȷʵ��ԭ���ǽⱾ��ؼ���֪��ʵ��������輰������������������������ѧ���ķ�����������ѧʵ��������

| ʵ���� | c��HA��/mol•L-1 | c��NaOH��/mol•L-1 | �����Һ��pH |
| I | 0.2 | 0.2 | pH=a |
| II | c1 | 0.2 | pH=7 |
| III | 0.2 | 0.1 | pH��7 |
| IV | 0.1 | 0.1 | pH=9 |
�ٲ�����������ʵ����������I��ʵ�������������a=7���������������=��������HAΪǿ�ᣮ
����II��ʵ��Ļ����Һ�У�c��A-��= c��Na+�����������������=������
�۲�����������ʵ����������III��ʵ�����������HA�����ᣨ�ǿ�����������������ӷ���ʽ��ʾ�����Һ�д��ڵ�����ƽ�⣺HA?H++A-��A-+H2O?HA+OH-��
��IV��ʵ��Ļ����Һ�У���ˮ�������c ��OH-��=10-5 mol/L��
| A�� | �۱�ϩ�Ľṹ��ʽ��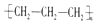 | B�� | ������ӵı���ģ�ͣ� | ||
| C�� | ��Ȳ�ĵ���ʽ�� | D�� | 2-�һ�-1��3-����ϩ���ӵļ���ʽ��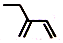 |
| ʵ������ | ʵ��Ŀ�� | |
| A | ��1mL 1%��NaOH��Һ�м���2mL 2%��CuSO4��Һ�����ټ���0.5mL�л���X��������к�δ����ש��ɫ���� | ��֤X�ṹ�в�����ȩ�� |
| B | �ڻ����������ӵı��еμ�������ˮ���������� | ��ȥ���������ı��� |
| C | ��������NaOH��Һ���������ٷֲ㣬��ȴ���ϡ���������ԣ��ٵμ�AgNO3��Һ | ��֤±��ԭ��Ϊ��ԭ�� |
| D | ���Ҵ���Ũ���Ṳ���Ƶõ�����ͨ������KMnO4��Һ�� | ���������к�����ϩ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ������ϵ���¶Ȼ�����ijһ��ֵ�Ũ�Ⱦ������ӷ�Ӧ��ϵ�л������ռ�ٷ��� | |
| B�� | H2+Cl2$\frac{\underline{\;��ȼ\;}}{\;}$2HCl��Ӧ�л�ѧ��ֻת��Ϊ���� | |
| C�� | �����������Ҫ���Ͻ��̻�����Ϊ����л��������С���ܱ���������ױ���ȼ��ը | |
| D�� | ����������ȷֽ���һ���ؼ�С�Ĺ��� |
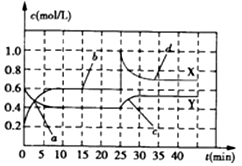
| A�� | ǰ10min����NO2��ʾ�Ļ�ѧ��Ӧ����v��NO2��=0.02mol/��L•min�� | |
| B�� | ��Ӧ������25minʱ�����߷����仯��ԭ�������������������NO2��g�� | |
| C�� | ��Ҫ�ﵽ�������ͬ�Ļ�ѧƽ��״̬����25minʱ�����Բ�ȡ�Ĵ�ʩ������N2O4��g�� | |
| D�� | a��b��c��d�ĸ����У���ʾ��ѧ��Ӧ����ƽ��״̬�ĵ����b��d |
| A�� |  ͼ�ɱ�ʾ��ƽ��N2��g��+3H2��g��?2NH3��g����ѹ��ͬʱ�Ƴ�����NH3ʱ�����ʱ仯 | |
| B�� |  ͼ��a��b����ֻ�ɱ�ʾ��ӦH2��g��ʮI2��g��?2HI��g�����д��������������½���ƽ��Ĺ��� | |
| C�� | 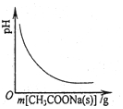 ͼ��ʾ��CH3COOH��Һ������CH3COONa�������ҺpH�ı仯 | |
| D�� | 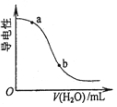 ͼ��ʾ�������Һ�м�ˮʱ�䵼���Ա仯����CH3COOH��Һ��pH��a��b |
