��Ŀ����
5��ij�������������Al����NH4��2SO4��MgCl2��FeCl2��AlCl3�е�һ�ֻ�����ɣ��ֶԸû����������ʵ�飬����������й�������ͼ��ʾ��������������ѻ���ɱ�״���µ��������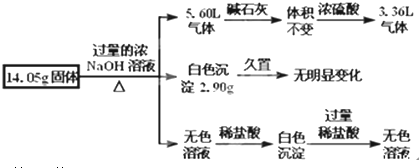
�ش��������⣺
��1����������Ƿ����FeCl2����ǡ�����
��2����������Ƿ���ڣ�NH4��2SO4�ǣ���ǡ������ж�����������ͨ��Ũ�������2.24L
��3����������Ƿ���AlCl3�ǣ���ǡ������ж����ݣ�����Ҫд�����������̣����ڵ�Al����NH4��2SO4��MgCl2��������С��14.05g��ʣ�����һ����AlCl3
��4��д������������з�Ӧ�����ӷ���ʽ�����м���д������NH4++OH-�TNH3��+H2O��2Al+2OH-+2H2O�T2AlO2-+3H2����Mg2++2OH-�TMg��OH��2����Al3++4OH-�TAlO2-+2H2O��
���� ������������ͨ����ʯ��������䣨���������壩����ͨ��Ũ���������С��˵��ʣ���3.36L����Ϊ��������ԭ������һ�����н���Al����������Ϊ��$\frac{3.36L}{22.4L/mol}$$\frac{2}{3}$��27 g/mol=2.7 g�����ɰ��������ʵ���Ϊ��$\frac{5.6L-3.36l}{22.4L/mol}$=0.1 mol����ԭ������һ������ 0.1 mol ��NH4��2SO4��������Ϊ6.6g���õ���ɫ�������ò���ɫ��˵����FeCl2�������������ױ�����Ϊ����ɫ����������������ΪNaOH����������ɫ���������ܺ���������������˵��2.90g��ɫ����ΪMg��OH��2��Ϊ0.05mol���������MgCl2Ϊ0.05mol������Ϊ4.75g����ɫ��Һ����Al�����NaOH��Һ��Ӧ��õ���NaAlO2���ݴ˽��н��
��� �⣺����ͼʾ��֪����������ͨ����ʯ��������䣨���������壩����ͨ��Ũ���������С��˵��ʣ���3.36L����Ϊ��������ԭ������һ�����н���Al����������Ϊ��$\frac{3.36L}{22.4L/mol}$$\frac{2}{3}$��27 g/mol=2.7 g�����ɰ��������ʵ���Ϊ��$\frac{5.6L-3.36l}{22.4L/mol}$=0.1 mol����ԭ������һ������ 0.1 mol ��NH4��2SO4��������Ϊ6.6g���õ���ɫ�������ò���ɫ��˵����FeCl2�������������ױ�����Ϊ����ɫ����������������ΪNaOH����������ɫ���������ܺ���������������˵��2.90g��ɫ����ΪMg��OH��2��Ϊ0.05mol���������MgCl2Ϊ0.05mol������Ϊ4.75g����ɫ��Һ����Al�����NaOH��Һ��Ӧ��õ���NaAlO2��
��1����ɫ�������ò���ɫ��˵����FeCl2�������������ױ�����Ϊ����ɫ��������������
�ʴ�Ϊ����
��2������ͨ��Ũ������������2.24L��˵����������NH3����ԭ������һ�����У�NH4��2SO4��
�ʴ�Ϊ���ǣ�����ͨ��Ũ�������2.24L��
��3�������Ϸ�����֪ԭ������һ������2.7g Al��ԭ������һ������ 0.05 mol ��NH4��2SO4��������Ϊ6.6g��������MgCl2Ϊ0.05mol������Ϊ4.75g���������ʵ�����֮�պõ���14.05g������һ��û��AlCl3��
�ʴ�Ϊ���ǣ����ڵ�Al����NH4��2SO4��MgCl2�� ������С��14.05g��ʣ�����һ����AlCl3��
��4������������з�Ӧ�����ӷ���ʽ�У�NH4++OH-�TNH3��+H2O��2Al+2OH-+2H2O�T2AlO2-+3H2����Mg2++2OH-�TMg��OH��2����Al3++4OH-�TAlO2-+2H2O��
�ʴ�Ϊ��NH4++OH-�TNH3��+H2O��2Al+2OH-+2H2O�T2AlO2-+3H2����Mg2++2OH-�TMg��OH��2����Al3++4OH-�TAlO2-+2H2O��
���� ���⿼�����ʵķ��롢�ᴿ�Լ����飬��Ŀ�Ѷ��еȣ�������Ԫ�ػ�����֪ʶ���ۺ�Ӧ�ã�Ϊ�߿����������ͣ�ע�����������ݽ����жϣ�����������ѧ���ķ�����������ѧ����������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ���ڦ� | B�� | ���ڦ� | C�� | С�ڦ� | D�� | ��ȷ�� |
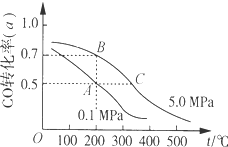
| A�� | A��B�����ʾ��ijʱ�̴ﵽƽ��״̬����ʱA��B����ʱ�����У�n��A���ܣ�n��B����=4��5 | |
| B�� | ��A��ʱ���ֺ��º��ݣ�������ϵ�г�������He�����´ﵽƽ��ǰv��������v���棩 | |
| C�� | ����ʼʱ�������г���1mol CO��2mol H2���ﵽƽ�⣨��A����ͬ�¶�ѹǿ��ʱ��CO�� ת����С��50% | |
| D�� | �ڲ��ı䷴Ӧ������������£����¡���ѹ�����״��ӻ����ϵ�з�������������CO��ת���� |
| A�� | H2SO4?2H++SO42- | B�� | BaCl2�TBa2++2Cl- | ||
| C�� | NH3•H2O�TNH4++OH- | D�� | Na2CO3�TNa++CO32- |
| A�� | ���Ϸ�Ӧһ����������ԭ��Ӧ | B�� | �ǽ���������һ�������������� | ||
| C�� | ����������һ���Ǽ��������� | D�� | ����������һ���ǽ��������� |
| A�� |  ��ȥ������Һ�еIJ����� | B�� | 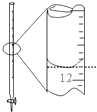 ��¼�ζ��յ����Ϊ12.20ml | ||
| C�� | 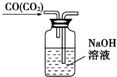 ��ȥCO�����е�CO2���� | D�� |  ���������Ʊ���ʾʵ�� |
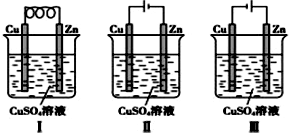
| A�� | ����ԭ��أ����ǵ��װ�� | |
| B�� | ��װ����п���Ͼ�����������Ӧ | |
| C�� | ��װ���У�ͭ��������������Ӧ���ܽ� | |
| D�� | ��װ����Cu2+Ũ�Ȼ������� |
| A�� | Fe������Cl2��ȼ������FeCl2 | |
| B�� | ʯ�͵��ѽ⡢ú�ĸ����ǻ�ѧ�仯 | |
| C�� | ��ѧ��Ӧ���ʱ��뷴Ӧ��;���й� | |
| D�� | ��������ͭ��a��b����;����ȫת��Ϊ����ͭ��;��a��b���ĵ�����һ���� ;��a��Cu$��_{��}^{O_{2}}$CuO$\stackrel{ϡ����}{��}$Cu��NO3��2��;��b��Cu$\stackrel{ϡ����}{��}$Cu��NO3��2 |
| A�� | ԭ����ǽ�����ת��Ϊ��ѧ�ܵ�װ�� | |
| B�� | ԭ�����������������Ӧ | |
| C�� | ��������������ԭ��Ӧ | |
| D�� | ���ص��������ӵ�Դ���� |