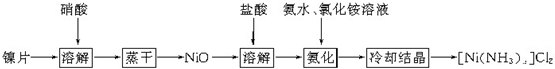
��Ŀ����
11��NaClO2�����ޡ��顢ճ����ά��֯���Ư�ף�ʵ�����Ʊ�NaClO2��װ����ͼ��ʾ��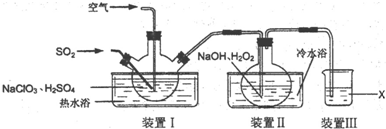
��1��װ��I�����¶���35��55�棬ͨ��SO2��NaClO3��ԭΪClO2���е㣺11�棩����Ӧ������ͨ�������Ŀ�������Ŀ���ǽ�ClO2���뵽װ�â���з�Ӧ��
��2��װ�â��з�Ӧ����NaClO2�Ļ�ѧ����ʽΪ2NaOH+2ClO2+H2O2=2NaClO2+2H2O+O2����Ӧ�����Һ�������ӳ���ClO2-����������ClO3-��Cl-��ClO-��OH-��������ܺ��е�һ����������SO42-�������ӷ���ʽ��ʾ�����Ӳ�����ԭ��ȡ������Ӧ�����Һ���ȼ����������ᣬ�ټ�BaCl2��Һ����������ɫ��������˵������SO42-��
��3����֪��NaClO2������Һ���¶ȵ���38��ʱ����������NaClO2•3H2O���¶ȸ���38��ʱ����������NaClO2���¶ȸ���60��ʱNaClO2�ֽ�����NaClO3��NaCl���벹���װ�â�Ӧ�����Һ�л��NaClO2����IJ������裮
�ټ�ѹ��55�������ᾧ���ڳ��ȹ��ˣ�����38�桫60����ˮϴ�ӣ��ܵ���60�����õ���Ʒ��
��4��װ�â����Լ�XΪNaOH��Һ��
���� ����ʵ��װ��ͼ��װ�â����ö�������ԭ���������ɶ������ȣ�װ�â�����˫��ˮ�ڼ��������»�ԭ������������NaClO2��װ�â�����������������β�����Է�ֹ��Ⱦ������
��1��װ�â��з�Ӧ����NaClO2����Ӧ������ͨ�������Ŀ�������ClO2���뵽װ�â���з�Ӧ��
��2��װ�â��з�Ӧ����NaClO2��ClԪ�صĻ��ϼ۽��ͣ�˫��ˮӦ���ֻ�ԭ�ԣ����������ɣ����ԭ���غ��֪������ˮ���ɣ���ƽ��д����ʽ��
װ��I��ͨ��SO2�IJ���ȫ��Ӧ����װ�â��б������������ᣬ�������������ᱵ�ǰ�ɫ���������������
��3������Һ����ȡ���壬һ����������ᾧ�����ˡ�ϴ�ӡ�����ķ�����ע���¶ȿ��ƣ�
��4��װ�â��е�����������β���п��ܴ��ڵĶ�������ClO2�ȣ���ֹ��Ⱦ����������������������Һ���գ�
��� �⣺��1��װ�â��з�Ӧ����NaClO2����Ӧ������ͨ�������Ŀ�������ClO2���뵽װ�â���з�Ӧ��
�ʴ�Ϊ����ClO2���뵽װ�â���з�Ӧ��
��2��װ�â��з�Ӧ����NaClO2��ClԪ�صĻ��ϼ۽��ͣ�˫��ˮӦ���ֻ�ԭ�ԣ����������ɣ����ԭ���غ��֪������ˮ���ɣ���ƽ��ʽΪ��2NaOH+2ClO2+H2O2=2NaClO2+2H2O+O2��װ��I��ͨ��SO2�IJ���ȫ��Ӧ����װ�â��б������������ᣬ��Һ�п��ܴ���SO42-���������Ȼ�����Һ����SO42-�����������ȡ������Ӧ�����Һ���ȼ����������ᣬ�ټ�BaCl2��Һ����������ɫ��������˵������SO42-��
�ʴ�Ϊ��2NaOH+2ClO2+H2O2=2NaClO2+2H2O+O2��SO42-��ȡ������Ӧ�����Һ���ȼ����������ᣬ�ټ�BaCl2��Һ����������ɫ��������˵������SO42-��
��3������Һ����ȡ���壬һ����������ᾧ�����ˡ�ϴ�ӡ�����ķ�����Ϊ��ֹ��������NaClO2•3H2O��Ӧ���ȹ��ˣ�����Ŀ��Ϣ��֪��Ӧ�����¶�38�桫60�����ϴ�ӣ�����60����
�ʴ�Ϊ�����ȹ��ˣ���38�桫60����ˮϴ�ӣ�����60����
��4��װ�â��е�����������β���п��ܴ��ڵĶ�������ClO2�ȣ�����������������Һ���գ���ֹ��Ⱦ������
�ʴ�Ϊ��NaOH��Һ��
���� ���⿼�����ʵ��Ʊ�������Ϣ�����á���װ�õ�����ȣ�����ԭ���ǽ���Ĺؼ���ͬʱ����ѧ���������⡢���������������ѶȽϴ�

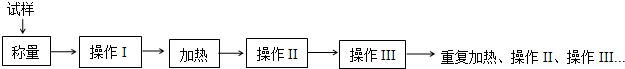
�ش��������⣺
��1��ʵ������������������ƽ���ƾ��ơ������żܣ�������������ѡ��ʵ�黹��Ҫ������A��B��E��F��G���ñ����ĸ��д��
A�������� B���в� C���Թܼ� D�������� E������ F�������� G��ҩ��
�������г��������⣬����Ҫ�������������ǡ�����ǯ��
��2��ijѧ����ʵ���еõ�����������ݴ˼��� x=5.9����ȷ��0.1��
| ����ǰ���� | ���Ⱥ����� | ||
| m1 | m2 | m3 | m4 |
| 5.400g | 7.900g | 6.900g | 6.901g |
a������ǰ����ʱ����δ��ȫ����
b��������μ��Ⱥ���������ϴ�
c�����Ⱥ�����δ�������������ȴ
d�����ȹ�������������ʧ��
| A�� | ���ܱ�ʾ��ѧ��Ӧ�а���ų��������ٵĻ�ѧ����ʽ | |
| B�� | �ܹ���ʾ��ѧ��Ӧ����ЧӦ�Ļ�ѧ����ʽ | |
| C�� | ��ЧӦ�Ĵ�С�뷴Ӧ���¶ȡ�ѹǿ�أ�ֻ�뷴Ӧ����������״̬�й� | |
| D�� | ���ಽ��Ӧ���Ȼ�ѧ����ʽ���ʱ�����Ӧ������Ҫ��� |
| A�� | �����¶ȣ�����Ӧ���ʼ�С������Ӧ�������� | |
| B�� | �����¶������������淴Ӧ�������Ӷ����̴ﵽƽ���ʱ�� | |
| C�� | �ﵽƽ��������¶Ȼ�����ѹǿ�������ڸ÷�Ӧƽ�������ƶ� | |
| D�� | �ﵽƽ������¶Ȼ��Сѹǿ�������ڸ÷�Ӧƽ�������ƶ� |
| ʱ��/min | 5 | 10 | 15 | 20 | 25 | 30 |
| C��NH3��/mol•L-1 | 0.08 | 0.14 | 0.18 | 0.20 | 0.20 | 0.20 |