��Ŀ����
7������500mL 0.5mol/L��NaOH��Һ���Իش��������⣺��1�����㣺��ҪNaOH���������Ϊ10.0g��
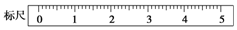
��2��ijѧ����������ƽ����һ��С�ձ�������������ǰ��������ڱ�ߵ���̶ȴ�����ƽ��ֹʱ����ָ���ڷֶ��̵�ƫ��λ�ã���ʱ��ߵ����̽�������ڡ����ڡ����ұߵ����̣���ʹ��ƽƽ�⣬�����еIJ���Ϊ����ߵ�ƽ����ĸ�����ƶ������ұߵ�ƽ����ĸ������ֱ����ƽƽ�⣮�ٶ����ճƵ�С�ձ�������Ϊ32.6���32.6g����31.61g���������á�������ʾ�������Ϸ������룬��������ʾ��������ȡ������ij������̣����ڱ���ϻ��������λ�ã�����������ʾ����
| ��������/g | 50 | 20 | 20 | 10 | 5 |
| ������ȡ��������̣� |
��3�����Ʒ������������������裺
����ʢ��NaOH���ձ��м���200mL����ˮʹ���ܽ⣬����ȴ�����£�
�ڼ���������ƿ�м�����ˮ��Һ��ӽ��̶���1��2cm��
�۽�NaOH��Һ�ز�����ע��500mL����ƿ�У�
�����ձ��м�������������ˮ��С��ϴ��2��3�κ���������ƿ��
�ݸ��ý�ͷ�ιܼ�����ˮ���̶��ߣ��Ӹ�ҡ�ȣ�
�Խ����ϲ����ų��Ⱥ�˳��٢ۢܢڢݣ�
��4��ijѧ��ʵ������NaOH��Һ��Ũ��Ϊ0.48mol/L��ԭ�������ACD��
A��ʹ����ֽ�����������ƹ��� B������ƿ��ԭ��������������ˮ
C���ܽ����ձ�δ�����ϴ�� D����ͷ�ιܼ�ˮ����ʱ���ӿ̶ȣ�
���� ��1������m=CVM�������ʵ�������
��2��������ƽ�ľ�ȷ��Ϊ0.1g���ֶ��̵�ָ��ƫ�ң�˵���ұ��أ�����ߵĺ�����˿���������ƽ�⣻����ʹ��������ƽʱ�����������ȷ������ɣ�
��3������һ�����ʵ���Ũ����Һһ�㲽��Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ��ݴ�����
��4���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=$\frac{n}{V}$�����жϣ�
��� �⣺��1������500mL 0.5mol/L��NaOH��Һ����Ҫ���ʵ�����m=0.5mol/L��0.5L��40g/mol=10.0g��
�ʴ�Ϊ��10.0g��
��2���ֶ��̵�ָ��ƫ�ң�˵���ұ��أ����̸������̣�Ӧ����ߵĺ�����˿������Ե���ƽ�⣮������ƽ�ľ�ȷ��Ϊ0.1g����ӦΪ32.6g�������ȼ�����������룬�ټ�С������ԭ����ѡ��50g���룬����ƫ���ٻ�20g���룬�����������㣬�ټ�20g���룬��������ƫ������10g���룬�����������㣬����5g���룬��������ƫ���ٵ������뵽2.2g��
�ʴ�Ϊ�����ڣ�����ߵ�ƽ����ĸ�����ƶ������ұߵ�ƽ����ĸ������ֱ����ƽƽ�⣻32.6g�� ��
��
��3������һ�����ʵ���Ũ����Һһ�㲽��Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ�������ȷ�IJ���˳��Ϊ���٢ۢܢڢݣ�
�ʴ�Ϊ���٢ۢܢڢݣ�
��4��ʵ������NaOH��Һ��Ũ��0.48mol•L-1��������ҺŨ��ƫ�ͣ�
A�� ����ֽ����ʱ����ֽ�������������ƣ�����ת���ձ�����������Ҳ���ˣ�������ҺŨ��ƫ�ͣ�
B�� �����Ҫ���ݣ�����ƿ�����������������ˮ������ҺŨ����Ӱ�죻
C�� δϴ��Һ�ձ�������������������ƿ���������Ƶ�ʵ��������С����ҺŨ��ƫ�ͣ�
D������ʱ���ӿ̶ȣ�ʹ��Һ�����ƫ��������ҺŨ��ƫ�ͣ�
��ѡ��ACD��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ�ע���c=$\frac{n}{V}$��������ԭ����ע�ⸯʴ���׳����ҩƷӦ���ڲ��������ڳ�����

 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�| A�� | 1.2mol | B�� | 2mol | C�� | 0.8mol | D�� | 1.8mol |

����˵���в���ȷ���ǣ�������
| A�� | ͼ����Y��ʾ��AԪ�صļ۵����� | |
| B�� | ͼ����Y��ʾ��AԪ���⻯��ķе� | |
| C�� | ͼ����Y��ʾ��������Ԫ�ص���������ϼ� | |
| D�� | ͼ����Y��ʾF-��Na+��Mg2+��Al3+�������ӵİ뾶 |
| ѡ�� | ʵ����ʵ | ���� |
| A | ����������ͬ��Na2S2O3��ҺŨ��Խ����ͬŨ�ȵ����ᷴӦ���������������ʱ��Խ�� | ��������������ʱ������Ӧ��Ũ�ȣ���ѧ��Ӧ�������� |
| B | ��ͭ���缫���CuSO4��Һ��CuSO4��ҺŨ�Ȳ��� | Cu2+û�в���缫��Ӧ |
| C | �ڳ�����N2������O2��Ӧ��������ȴ����ȼ | ��Ԫ�صķǽ����Ա���Ԫ�ص��� |
| D | ��������Ӧ | �������һ�����ܴ����ڼ�����Һ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| X | Y | ||
| Z | W |
| A�� | Ԫ�ص�����ϼ�Z����Y | |
| B�� | Ԫ�ص���̬�⻯����ȶ���Wǿ��Y | |
| C�� | ԭ�Ӱ뾶Z��YС | |
| D�� | Ԫ�ص�����������ˮ��������X����W |
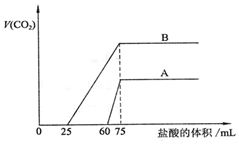 ȡ�����ʵ���Ũ�ȵ�NaOH��Һ����A��B��ÿ��50mL�������и�ͨ��һ������CO2��Ȼ���ȡ��Һ10mL���ֱ���ϡ��Ϊ100mL���ٷֱ���ϡ�ͺ����Һ����μ���0.1mol/L�����ᣬ��״���²�����CO2��������������������֮��Ĺ�ϵ��ͼ��ʾ���Իش��������⣺
ȡ�����ʵ���Ũ�ȵ�NaOH��Һ����A��B��ÿ��50mL�������и�ͨ��һ������CO2��Ȼ���ȡ��Һ10mL���ֱ���ϡ��Ϊ100mL���ٷֱ���ϡ�ͺ����Һ����μ���0.1mol/L�����ᣬ��״���²�����CO2��������������������֮��Ĺ�ϵ��ͼ��ʾ���Իش��������⣺