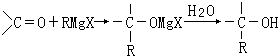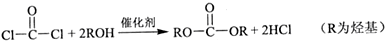��Ŀ����
��1���Ȼ���ˮ��Һ�� �Ե�ԭ���ǣ������ӷ���ʽ��ʾ���� ����AlCl3��Һ���ɣ����գ����õ�����Ҫ��������� ��
��2��pH=3�Ĵ����pH=11������������Һ�������Ϻ���Һ�� �ԣ���Һ��c��Na+��
c��CH3COO-�� ���������=����������
��3��ijѧ����0.100mol?L-1��KOH����Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷֽ�Ϊ���¼�����
A����ȡ20.00mL����������Һע��ྻ����ƿ��������2��3�η�̪��
B���ñ���Һ��ϴ�ζ���2��3�Σ�
C����ʢ�б���Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ��
D��ȡ��KOH��Һע���ʽ�ζ������̶ȡ�0������2��3mL��
E������Һ������0����0�����¿̶ȣ����¶�����
F������ƿ���ڵζ��ܵ����棬�ñ�KOH��Һ�ζ����յ㲢���µζ���Һ��Ŀ̶ȣ�
�ʹ�ʵ�������գ�
����ȷ���������˳���ǣ�����ĸ�����д�� ��
������A�������֮ǰ�������ô�����Һ��ϴ��ƿ����ζ���� ���ƫ�ߡ���ƫ�͡����䡱����
���жϵ���ζ��յ��ʵ�������� ��
��2��pH=3�Ĵ����pH=11������������Һ�������Ϻ���Һ��
c��CH3COO-�� ���������=����������
��3��ijѧ����0.100mol?L-1��KOH����Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷֽ�Ϊ���¼�����
A����ȡ20.00mL����������Һע��ྻ����ƿ��������2��3�η�̪��
B���ñ���Һ��ϴ�ζ���2��3�Σ�
C����ʢ�б���Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ��
D��ȡ��KOH��Һע���ʽ�ζ������̶ȡ�0������2��3mL��
E������Һ������0����0�����¿̶ȣ����¶�����
F������ƿ���ڵζ��ܵ����棬�ñ�KOH��Һ�ζ����յ㲢���µζ���Һ��Ŀ̶ȣ�
�ʹ�ʵ�������գ�
����ȷ���������˳���ǣ�����ĸ�����д��
������A�������֮ǰ�������ô�����Һ��ϴ��ƿ����ζ����
���жϵ���ζ��յ��ʵ��������
���㣺�к͵ζ�,����ˮ���ԭ��,�����ʱ�Ķ����жϼ��й�ph�ļ���
ר�⣺
��������1���Ȼ���Ϊǿ�������Σ�������ˮ���ʹ����Һ�����ԣ�AlCl3Ϊǿ�������Σ����ȴٽ�ˮ�������������������ᣬ�����ӷ������������ɷֽ⣻
��2��pH=3�Ĵ��ᣬ��Ũ�ȴ���0.001mol/L��pH=11���������ƣ���Ũ�ȵ���0.001mol/L���������Ϻ�����������ݵ���غ��֪��c��Na+��+c��H+��=c��OH-��+c��CH3COO-���������������Һ�����ԣ�����c��H+����C��OH-����
��3�����к͵ζ��м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ƿ��Ȼ�����ָʾ�����еζ��Ȳ�����
�ڸ���c���ᣩ=
�жϲ��������������������Ӱ�죻
������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
��2��pH=3�Ĵ��ᣬ��Ũ�ȴ���0.001mol/L��pH=11���������ƣ���Ũ�ȵ���0.001mol/L���������Ϻ�����������ݵ���غ��֪��c��Na+��+c��H+��=c��OH-��+c��CH3COO-���������������Һ�����ԣ�����c��H+����C��OH-����
��3�����к͵ζ��м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ƿ��Ȼ�����ָʾ�����еζ��Ȳ�����
�ڸ���c���ᣩ=
| c(��)��V(��) |
| V(��) |
������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
���
�⣺��1��AlCl3Ϊǿ�������Σ����ȴٽ�ˮ�������������������ᣬˮ�ⷽ��ʽΪAl3++3H2O?Al��OH��3+3H+�������ӷ������������ɷֽ�����Al2O3��
�ʴ�Ϊ���Al3++3H2O?Al��OH��3+3H+��Al2O3��
��2��pH=3�Ĵ��ᣬ��Ũ�ȴ���0.001mol/L��pH=11���������ƣ���Ũ�ȵ���0.001mol/L���������Ϻ��������Ϊ����ʹ����ƵĻ����Һ������Һ�����ԣ������������Һ�����ԣ�����c��H+����C��OH-�������ݵ���غ��֪��c��Na+��+c��H+��=c��OH-��+c��CH3COO-��������c��Na+����c��CH3COO-�����ʴ�Ϊ�������
��3���ٲ����IJ�����ѡ��ζ��ܣ�Ȼ��ϴ�ӡ�װҺ��ʹ���������Һ���̶��ڵζ�̨�ϣ�Ȼ�����Һ����¶�������ȡ����Һ����ƿ��Ȼ�����ָʾ�����еζ�������˳��Ϊ��B��D��C��E��A��F��
�ʴ�Ϊ��B��D��C��E��A��F��
����ƿ������ˮϴ�Ӻ�������ô���Һ��ϴ����ʹ��ƿ�����ʵ����ʵ����������V���ƫ����c���ᣩ=
�жϻ���ɽ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
�ۣ�4����ʵ������NaOH�ζ�������Һ���÷�̪��ָʾ���������յ�ʱ�������ǵ���Һ����ɫ��Ϊdz��ɫ�����ڰ�����ڲ���ɫ��
�ʴ�Ϊ������Һ����ɫ��Ϊdz��ɫ�����ڰ�����ڲ���ɫ��
�ʴ�Ϊ���Al3++3H2O?Al��OH��3+3H+��Al2O3��
��2��pH=3�Ĵ��ᣬ��Ũ�ȴ���0.001mol/L��pH=11���������ƣ���Ũ�ȵ���0.001mol/L���������Ϻ��������Ϊ����ʹ����ƵĻ����Һ������Һ�����ԣ������������Һ�����ԣ�����c��H+����C��OH-�������ݵ���غ��֪��c��Na+��+c��H+��=c��OH-��+c��CH3COO-��������c��Na+����c��CH3COO-�����ʴ�Ϊ�������
��3���ٲ����IJ�����ѡ��ζ��ܣ�Ȼ��ϴ�ӡ�װҺ��ʹ���������Һ���̶��ڵζ�̨�ϣ�Ȼ�����Һ����¶�������ȡ����Һ����ƿ��Ȼ�����ָʾ�����еζ�������˳��Ϊ��B��D��C��E��A��F��
�ʴ�Ϊ��B��D��C��E��A��F��
����ƿ������ˮϴ�Ӻ�������ô���Һ��ϴ����ʹ��ƿ�����ʵ����ʵ����������V���ƫ����c���ᣩ=
| c(��)��V(��) |
| V(��) |
�ʴ�Ϊ��ƫ�ߣ�
�ۣ�4����ʵ������NaOH�ζ�������Һ���÷�̪��ָʾ���������յ�ʱ�������ǵ���Һ����ɫ��Ϊdz��ɫ�����ڰ�����ڲ���ɫ��
�ʴ�Ϊ������Һ����ɫ��Ϊdz��ɫ�����ڰ�����ڲ���ɫ��
���������⿼��������ˮ�⣬�������Һ�Ķ��Է���������к͵ζ��IJ�����������ķ����ȣ�ע�����غ㡢����ˮ������ã�

��ϰ��ϵ�д�
�����Ŀ
��NAΪ�����ӵ���������ֵ������˵����ȷ���ǣ�������
| A��5.6g����������Cl2��Ӧ��ʧȥ�ĵ�����Ϊ0.2NA |
| B�����³�ѹ�£�18g H2O����10NA������ |
| C�������£�1L 0.1mol/L AlCl3��Һ�к�Al3+��Ϊ0.1NA |
| D��1 mol Cu������Ũ���ᷴӦ����2NA��SO2���� |
�ñ�����ζ�δ֪Ũ�ȵ�NaOH��Һ�������ý��ƫ�ͣ����������ԭ����������������еģ�������
| A���ζ������У���ƿ������Һ���� |
| B����ƿ������ˮϴ����δ�����T���еζ� |
| C����ʽ�ζ���δ�ñ�������ϴ |
| D���ζ�ǰ��ʽ�ζ��ܼ��첿�������ݣ��ζ���ֹʱ������ʧ |
�ñ�������ζ�δ֪Ũ�ȵ�NaOH��Һ�����в�����������ʵ�������ǣ�������
| A��������ˮϴ����ʽ�ζ��ܺ�װ���������еζ� |
| B��������ˮϴ����ƿ������NaOH��Һ��ϴ����װ��NaOH��Һ���еζ� |
| C���ü�ʽ�ζ���ȡ10.00 mL NaOH��Һ����������ˮϴ������ƿ�У��ټ�����������ˮ���еζ� |
| D���÷�̪��ָʾ��������ɫ�ձ���ɫʱ��ֹͣ������ |

 ����һ�������¿��Է�����ͼ��ʾ��ת�������������ˮ����ȥ����
����һ�������¿��Է�����ͼ��ʾ��ת�������������ˮ����ȥ����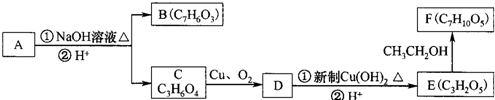
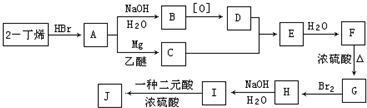
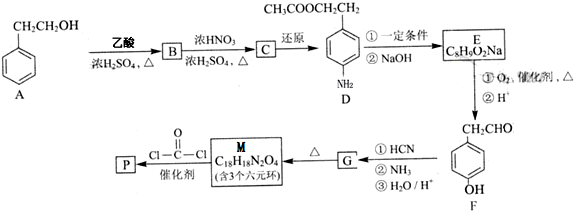
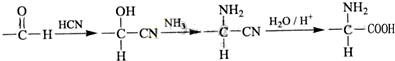
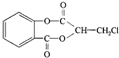
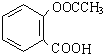 ��������ˮ���ᣬ����ʽ C9H8O4�����������������İ�˾ƥ�ֵ�ͬ���칹����
��������ˮ���ᣬ����ʽ C9H8O4�����������������İ�˾ƥ�ֵ�ͬ���칹���� �����������л���Ӧ�Ƶã���д��������Ϊ��Ҫԭ���Ʊ��л���C�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�CH3CH2OH
�����������л���Ӧ�Ƶã���д��������Ϊ��Ҫԭ���Ʊ��л���C�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�CH3CH2OH