��Ŀ����
���㻯����A��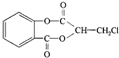 ����һ�������¿��Է�����ͼ��ʾ��ת�������������ˮ����ȥ����
����һ�������¿��Է�����ͼ��ʾ��ת�������������ˮ����ȥ����
��ش��������⣺
��1��д��A�ķ���ʽ ��
��2��1mol A������������Һ�м��ȣ���ַ�Ӧ������������ mol��
��3��д����E�Ƶ�F�Ļ�ѧ����ʽ�� ����Ӧ������ ��
��4���л���B����������CH3COOCOCH3��ֱ�ӷ�Ӧ���Ƶð�˾ƥ�֣�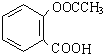 ��������ˮ���ᣬ����ʽ C9H8O4�����������������İ�˾ƥ�ֵ�ͬ���칹���� �֣�
��������ˮ���ᣬ����ʽ C9H8O4�����������������İ�˾ƥ�ֵ�ͬ���칹���� �֣�
�ٱ�����ֻ������������ ������������ ���ܷ���������Ӧ
���к˴Ź���������3��2��2��1�Ľṹ��ʽ�� �֣�
��5���л���C���������ᣨ �����������л���Ӧ�Ƶã���д��������Ϊ��Ҫԭ���Ʊ��л���C�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�CH3CH2OH
�����������л���Ӧ�Ƶã���д��������Ϊ��Ҫԭ���Ʊ��л���C�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�CH3CH2OH
CH2=CH2
CH2CH3CH3��
 ����һ�������¿��Է�����ͼ��ʾ��ת�������������ˮ����ȥ����
����һ�������¿��Է�����ͼ��ʾ��ת�������������ˮ����ȥ����
��ش��������⣺
��1��д��A�ķ���ʽ
��2��1mol A������������Һ�м��ȣ���ַ�Ӧ������������
��3��д����E�Ƶ�F�Ļ�ѧ����ʽ��
��4���л���B����������CH3COOCOCH3��ֱ�ӷ�Ӧ���Ƶð�˾ƥ�֣�
 ��������ˮ���ᣬ����ʽ C9H8O4�����������������İ�˾ƥ�ֵ�ͬ���칹����
��������ˮ���ᣬ����ʽ C9H8O4�����������������İ�˾ƥ�ֵ�ͬ���칹�����ٱ�����ֻ������������ ������������ ���ܷ���������Ӧ
���к˴Ź���������3��2��2��1�Ľṹ��ʽ��
��5���л���C���������ᣨ
 �����������л���Ӧ�Ƶã���д��������Ϊ��Ҫԭ���Ʊ��л���C�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�CH3CH2OH
�����������л���Ӧ�Ƶã���д��������Ϊ��Ҫԭ���Ʊ��л���C�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�CH3CH2OH | ŨH2SO4 |
| 170�� |
| H2 |
| ����/�� |
���㣺�л���ĺϳ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
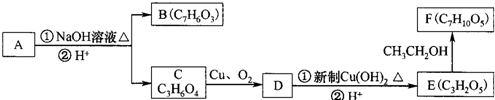
������AΪ ������������ˮ��Һ�����·���ˮ�ⷴӦ���ữ�õ�B��C�����B��C����ʽ��֪��BΪ
������������ˮ��Һ�����·���ˮ�ⷴӦ���ữ�õ�B��C�����B��C����ʽ��֪��BΪ ��CΪHOCH2CH��OH��COOH����ת����ϵ��֪��DΪ
��CΪHOCH2CH��OH��COOH����ת����ϵ��֪��DΪ ��EΪ
��EΪ ��E���Ҵ�����ת����Ӧ�õ�FΪ
��E���Ҵ�����ת����Ӧ�õ�FΪ ���ݴ˽��
���ݴ˽��
 ������������ˮ��Һ�����·���ˮ�ⷴӦ���ữ�õ�B��C�����B��C����ʽ��֪��BΪ
������������ˮ��Һ�����·���ˮ�ⷴӦ���ữ�õ�B��C�����B��C����ʽ��֪��BΪ ��CΪHOCH2CH��OH��COOH����ת����ϵ��֪��DΪ
��CΪHOCH2CH��OH��COOH����ת����ϵ��֪��DΪ ��EΪ
��EΪ ��E���Ҵ�����ת����Ӧ�õ�FΪ
��E���Ҵ�����ת����Ӧ�õ�FΪ ���ݴ˽��
���ݴ˽�����
�⣺AΪ ������������ˮ��Һ�����·���ˮ�ⷴӦ���ữ�õ�B��C�����B��C����ʽ��֪��BΪ
������������ˮ��Һ�����·���ˮ�ⷴӦ���ữ�õ�B��C�����B��C����ʽ��֪��BΪ ��CΪHOCH2CH��OH��COOH����ת����ϵ��֪��DΪ
��CΪHOCH2CH��OH��COOH����ת����ϵ��֪��DΪ ��EΪ
��EΪ ��E���Ҵ�����ת����Ӧ�õ�FΪ
��E���Ҵ�����ת����Ӧ�õ�FΪ ��
��
��1������A�Ľṹ��ʽ��֪A�ķ���ʽΪC10H7O4Cl��
�ʴ�Ϊ��C10H7O4Cl��
��2����ΪA��������������һ��±ԭ�ӣ�����һ������ˮ������ɷ��ǻ�������1mol A������������Һ�м��ȣ���ַ�Ӧ������������ 4mol��
�ʴ�Ϊ��4��
��3����E�Ƶ�F�Ļ�ѧ����ʽΪ�� ����Ӧ������ȡ������������Ӧ��
����Ӧ������ȡ������������Ӧ��
�ʴ�Ϊ�� ��ȡ������������Ӧ��
��ȡ������������Ӧ��
��4���������������İ�˾ƥ�֣� ����ͬ���칹��ٱ�����ֻ�������������������������������ܷ���������Ӧ��������һ�������γɵ�����������Ϊ-OOCH��-OOCCH3����-OOCH��-COOCH3����-OOCH��-CH2OOCH�������ڡ��䡢�����֣��ʹ���9�֣����к˴Ź���������3��2��2��1�Ľṹ��ʽΪ
����ͬ���칹��ٱ�����ֻ�������������������������������ܷ���������Ӧ��������һ�������γɵ�����������Ϊ-OOCH��-OOCCH3����-OOCH��-COOCH3����-OOCH��-CH2OOCH�������ڡ��䡢�����֣��ʹ���9�֣����к˴Ź���������3��2��2��1�Ľṹ��ʽΪ ��
�� ��2�֣�
��2�֣�
�ʴ�Ϊ��9��2��
��5���л���CΪHOCH2CH��OH��COOH�������ᣨ ���Ƶ�C����������ȥ�ټӳ�������һ�������ţ�������ȥ����̼̼˫����������ӳɣ���ˮ�⼴�ò�Ʒ������ϳ�·��Ϊ
���Ƶ�C����������ȥ�ټӳ�������һ�������ţ�������ȥ����̼̼˫����������ӳɣ���ˮ�⼴�ò�Ʒ������ϳ�·��Ϊ ��
��
�ʴ�Ϊ�� ��
��
 ������������ˮ��Һ�����·���ˮ�ⷴӦ���ữ�õ�B��C�����B��C����ʽ��֪��BΪ
������������ˮ��Һ�����·���ˮ�ⷴӦ���ữ�õ�B��C�����B��C����ʽ��֪��BΪ ��CΪHOCH2CH��OH��COOH����ת����ϵ��֪��DΪ
��CΪHOCH2CH��OH��COOH����ת����ϵ��֪��DΪ ��EΪ
��EΪ ��E���Ҵ�����ת����Ӧ�õ�FΪ
��E���Ҵ�����ת����Ӧ�õ�FΪ ��
����1������A�Ľṹ��ʽ��֪A�ķ���ʽΪC10H7O4Cl��
�ʴ�Ϊ��C10H7O4Cl��
��2����ΪA��������������һ��±ԭ�ӣ�����һ������ˮ������ɷ��ǻ�������1mol A������������Һ�м��ȣ���ַ�Ӧ������������ 4mol��
�ʴ�Ϊ��4��
��3����E�Ƶ�F�Ļ�ѧ����ʽΪ��
 ����Ӧ������ȡ������������Ӧ��
����Ӧ������ȡ������������Ӧ���ʴ�Ϊ��
 ��ȡ������������Ӧ��
��ȡ������������Ӧ����4���������������İ�˾ƥ�֣�
 ����ͬ���칹��ٱ�����ֻ�������������������������������ܷ���������Ӧ��������һ�������γɵ�����������Ϊ-OOCH��-OOCCH3����-OOCH��-COOCH3����-OOCH��-CH2OOCH�������ڡ��䡢�����֣��ʹ���9�֣����к˴Ź���������3��2��2��1�Ľṹ��ʽΪ
����ͬ���칹��ٱ�����ֻ�������������������������������ܷ���������Ӧ��������һ�������γɵ�����������Ϊ-OOCH��-OOCCH3����-OOCH��-COOCH3����-OOCH��-CH2OOCH�������ڡ��䡢�����֣��ʹ���9�֣����к˴Ź���������3��2��2��1�Ľṹ��ʽΪ ��
�� ��2�֣�
��2�֣��ʴ�Ϊ��9��2��
��5���л���CΪHOCH2CH��OH��COOH�������ᣨ
 ���Ƶ�C����������ȥ�ټӳ�������һ�������ţ�������ȥ����̼̼˫����������ӳɣ���ˮ�⼴�ò�Ʒ������ϳ�·��Ϊ
���Ƶ�C����������ȥ�ټӳ�������һ�������ţ�������ȥ����̼̼˫����������ӳɣ���ˮ�⼴�ò�Ʒ������ϳ�·��Ϊ ��
���ʴ�Ϊ��
 ��
��
���������⿼���л����ƶϺ��л��ϳɣ�ע���������л���ṹ��ʽ�����ʽ��ת�����������ƶϣ�ע���л��ϳ��й����ŵ����뷽�����Ѷ��еȣ�

��ϰ��ϵ�д�
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
�����Ŀ
�������ӷ���ʽ�У�����ˮ�ⷴӦ���ǣ�������
| A��CH3COOH+H2O?CH3COO-+H3O+ |
| B��SO2+H2O?HSO3-+H+ |
| C��H2O+H2O?H3O++OH- |
| D��NH4++H2O?NH3��H2O+H+ |



