��Ŀ����
11��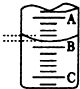 ���ᣨH2C2O4����������Ȼ���ֲ���У���K1=5.4��10-2��K2=5.4��10-5�����л�ԭ�ԣ�����ˮ����Һ�����ԣ�Ϊ�ⶨijH2C2O4��Һ��Ũ�ȣ�ȡ����Һ����ƿ�У���������ϡH2SO4����Ũ��Ϊc mol/L KMnO4����Һ�ζ����ζ�ԭ��Ϊ��2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O
���ᣨH2C2O4����������Ȼ���ֲ���У���K1=5.4��10-2��K2=5.4��10-5�����л�ԭ�ԣ�����ˮ����Һ�����ԣ�Ϊ�ⶨijH2C2O4��Һ��Ũ�ȣ�ȡ����Һ����ƿ�У���������ϡH2SO4����Ũ��Ϊc mol/L KMnO4����Һ�ζ����ζ�ԭ��Ϊ��2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O��1���ζ�����ʹ��֮ǰ��������еIJ����Ǽ���Ƿ�©ˮ���ζ�ʱ��KMnO4��ҺӦװ����ʽ�ζ��ܣ����ʽ�ζ��ܡ���ʽ�ζ��ܡ����У��ﵽ�ζ��յ�ʱ������Ϊ�������һ��KMnO4��Һ����Һ����ɫ��Ϊdz�Ϻ�ɫ���Ұ���Ӳ���ɫ��
��2����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��C���Ŀ̶�Ϊ20���ζ�����Һ�����ӦΪ19.40mL����ʱ�ζ�����Һ����������30.60mL��������ڡ�С�ڻ���ڣ�
��3��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡH2C2O4��Һ�����ΪVmL������ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����KMnO4��Һ���/mL | 22.32 | 24.39 | 24.41 |
A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱKMnO4��Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
C����һ�εζ�ʢװ��Һ�ĵζ���װҺǰ������ˮ��ϴ����δ�ñ�Һ��ϴ��
D����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ��
E���μ�KMnO4��Һ���죬δ������տ�����Һ��ɫ������ֹͣ�ζ�
��4�������������ݣ�д��H2C2O4�����ʵ���Ũ�ȵı���ʽ�����뻯��C=$\frac{61c}{V}$mol/L��
��5������һ����Ƽ�ʵ��֤�����������ǿ��̼�ᣬʵ�������������ȡ������NaHCO3���Թ��У����������Һ�������ݲ�����
���� ��1��ʵ��ǰ�ζ��ܱ�����м�©��KMnO4��Һ����ǿ�����ԣ����Ը�ʴ��Ƥ�ܣ�KMnO4��Һ����ɫ�����ᷴӦ��ϣ��������һ��KMnO4��Һ����Һ����ɫ��Ϊdz�Ϻ�ɫ���Ұ���Ӳ���ɫΪ�ζ����յ㣻
��2����A��C�̶ȼ����1mL��˵��ÿ����С��֮����0.1mL��C���Ŀ̶�Ϊ20���ݴ�ȷ��A�Ŀ̶ȣ�ע��ζ��ܵ�������ֵС��������ֵ�������һ�ξ���û�п̶ȣ�
��3���������ù����жϲ��������������������Ӱ�죻
��4�����ݻ�ѧ����ʽ2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O��������Ũ�ȣ�
��5������ǿ���������ԭ����ǿ���������η�Ӧ�����
��� �⣺��1���ζ�����ʹ��֮ǰ��������еIJ����Ǽ���Ƿ�©ˮ��KMnO4��Һ����ǿ�����ԣ����Ը�ʴ��Ƥ�ܣ���KMnO4��ҺӦװ����ʽ�ζ����У�
KMnO4��Һ����ɫ�����ᷴӦ��ϣ��������һ��KMnO4��Һ����Һ����ɫ��Ϊdz�Ϻ�ɫ���Ұ���Ӳ���ɫΪ�ζ����յ㣬
�ʴ�Ϊ�����ʽ�ζ��ܣ��������һ��KMnO4��Һ����Һ����ɫ��Ϊdz�Ϻ�ɫ���Ұ���Ӳ���ɫ��
��2����A��C�̶ȼ����1mL��˵��ÿ����С��֮����0.1mL��C���Ŀ̶�Ϊ20��A��B֮�����ĸ�С���������0.40mL����B��19.40mL�����ڵζ���50.00mL�̶��·�����Һ�壬����ʵ����Һ��Һ�����50mL-19.40mL=30.60mL��
�ʴ�Ϊ��19.40�����ڣ�
��3��A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱKMnO4��Һ�������Һ��ƫ�ߣ�����ƫС������KMnO4���ƫС����A��ȷ��
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ����������ݣ�����KMnO4���ƫ��B����
C����һ�εζ�ʢװ��Һ�ĵζ���װҺǰ������ˮ��ϴ����δ�ñ�Һ��ϴ����Һ��ϡ�ͣ�KMnO4Ũ��ƫС������KMnO4���ƫ��C����
D����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ����ϴ��ƿ���²�������ʵ���ƫ����KMnO4���ƫ��D����
E���μ�KMnO4��Һ���죬δ������տ�����Һ��ɫ������KMnO4���ƫС����E��ȷ��
��ѡ��AE��
��4�����εζ�����KMnO4��Һ����ֱ�Ϊ��22.32mLmL��24.39mL��24.41mL����һ�����ϴ���ȥ��mLmLmL����KMnO4��Һƽ�����Ϊ$\frac{24.39mL+24.41mL}{2}$=24.40mL������KMnO4���ʵ���Ϊn��KMnO4��=cmol/L��0.0244L����2KMnO4+5H2C2O4+3H2SO4�TK2SO4+2MnSO4+10CO2��+8H2O��֪��n��H2C2O4��=$\frac{5}{2}$n��KMnO4��=$\frac{5}{2}$��cmol/L��0.0244L=0.061cmol��������Ũ��Ϊ$\frac{0.061cmol}{V��1{0}^{-3}L}$=$\frac{61c}{V}$mol/L��
�ʴ�Ϊ��$\frac{61c}{V}$mol/L��
��5��ȡ������NaHCO3���Թ��У����������Һ�������ݲ�����֤�����������ǿ��̼�
�ʴ�Ϊ��ȡ������NaHCO3���Թ��У����������Һ�������ݲ�����
���� ���������ʺ���Ϊ��������������ԭ�ζ�����㣬�Ѷ��еȣ�ע��ʵ��ԭ�������գ��ζ����ϵĿ̶Ⱥ���Ͳ�Ͽ̶ȵ�����Ϊ�״��㣮

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ϡ����������������Һ��Ӧ��H++OH-=H2O | |
| B�� | ����ϡ���ᷴӦ��Al+2H+=Al3++H2�� | |
| C�� | ���Ȼ�����Һ������������Һ��Ӧ��FeCl3+3OH-=Fe��OH��3��+3C1- | |
| D�� | ������̼�����ʯ��ˮ��Ӧ��CO2+2OH-=CO32-+H2O |
| A�� | SiO2$\stackrel{HCl}{��}$SiCl4$\stackrel{H_{2}}{��}$Si | |
| B�� | MgCO3$\stackrel{HCl}{��}$MgCl2��Һ$\stackrel{���}{��}$Mg | |
| C�� | Fe$��_{��ȼ}^{O_{2}}$Fe2O3$\stackrel{H_{2}SO_{4}}{��}$Fe2��SO4��3 | |
| D�� | Na$��_{��ȼ}^{O_{2}}$Na2O2$\stackrel{CO_{2}}{��}$Na2CO3 |
ʵ��һ����ͬѧ�о���ʵ�鱨�������
| ʵ �� �� �� | �� �� | �� �� |
| �ٷֱ�ȡ�������2mol/L�������Թ��У� �ڷֱ�Ͷ���С����״��ͬ��Cu��Fe��Mg�� | ��Ӧ������ Mg��Fe��Cu | ��Ӧ�������Խ���ã���Ӧ����Խ�죮 |
ʵ�������֪2KMnO4+5H2C2O4+3H2SO4�TK2SO4+2MnSO4+8H2O+10CO2�����ڸ������������Һ�Ͳ�����Һ��Ӧʱ�����ֿ�ʼһ��ʱ�䷴Ӧ���ʽ�������Һ��ɫ�����ԣ�������ͻȻ��ɫ����Ӧ�������Լӿ죮
��1���������ʵ������ijͬѧ��ΪKMnO4��H2C2O4��Ӧ�Ƿ��ȷ�Ӧ��������Һ�¶����ߣ���Ӧ���ʼӿ죮��Ӱ�컯ѧ��Ӧ���ʵ����ؿ�������뷴Ӧ�������Լӿ��ԭ���������ɵ��������д����ã�
��2������ʵ��֤����IJ��룬�����Ը��������Һ��������Һ�⣬����Ҫѡ����Լ����������B��ѡ���ţ���
A������� B�������� C��ˮ D���Ȼ��̣�
��1������к͵ζ�--�ñ�����ζ�δ֪Ũ�ȵ�NaOH��Һ�����в�����ɲⶨ���ƫ�ߵ���CD ����ѡ����ĸ��
A���ζ��յ����ʱ�����ӵζ��̶ܿȣ�����������ȷ��
B��ʢװδ֪Һ����ƿ������ˮϴ����δ��δ֪Һ��ϴ
C����ʽ�ζ���������ˮϴ����δ�ñ�������ϴ
D���ζ�ǰ���ζ��ܼ��������ݣ��ζ���������ʧ
��2��������ԭ�ζ�--ȡ������Һ������ƿ�У���������ϡ���ᣬ��Ũ��Ϊ0.1mol•L-1�ĸ��������Һ�ζ��������ķ�ӦΪ��
2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O�������м�¼��ʵ�����ݣ�
| �ζ����� | ����Һ�����mL�� | ��KMnO4��Һ�����mL�� | |
| �ζ�ǰ���� | �ζ������ | ||
| ��һ�� | 25.00 | 0.50 | 20.40 |
| �ڶ��� | 25.00 | 3.00 | 23.00 |
| ������ | 25.00 | 4.00 | 24.10 |
�ڸò�����Һ�����ʵ���Ũ��Ϊ0.2 mol•L-1��
��3�������ζ�--�ζ����ͱ��ζ����������ȵζ�����ָʾ��������������ܣ�
�ο��±��е����ݣ�����AgNO3�ζ�NaSCN��Һ����ѡ�õ�ָʾ����D
����ѡ����ĸ����
| ������ | AgCl | AgBr | AgCN | Ag2CrO4 | AgSCN |
| ��ɫ | �� | dz�� | �� | ש�� | �� |
| Ksp | 1.77��10-10 | 5.35��10-13 | 1.21��10-16 | 1.12��10-12 | 1.0��10-12 |
| ʵ���� | ��Ӧ�� | ���� |
| �� | 10mL2% H2O2��Һ | �� |
| �� | 10mL5% H2O2��Һ | �� |
| �� | 10mL5% H2O2��Һ | 1mL0.1mol•L-1FeCl3��Һ |
| �� | 10mL5% H2O2��Һ+����HCl��Һ | 1mL0.1mol•L-1FeCl3��Һ |
| �� | 10mL5% H2O2��Һ+����NaOH��Һ | 1mL0.1mol•L-1FeCl3��Һ |
��2��������5% H2O2��Һ��pHԼΪ6��H2O2�ĵ��뷽��ʽΪ⇒H2O2?H++HO2-��
��3��ʵ��ٺ͢ڵ�Ŀ����̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죮
��4��ʵ��ۡ��ܡ����У�������������������ʱ��仯�Ĺ�ϵ��ͼ��
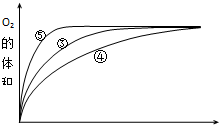
������ͼ�ܹ��ó���ʵ������Ǽ��Ի���������H2O2�ֽ�����ʣ����Ի����ܼ�СH2O2�ֽ�����ʣ�
| A�� | ͭƬ��ʯī�����Ҵ� | B�� | ͭƬ��ʯī������������Һ | ||
| C�� | пƬ��ͭƬ��ϡ���� | D�� | ͭƬ����Ƭ��FeCl3��Һ |
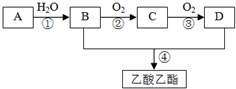 A��ʯ���ѽ�������Ҫ�ɷ֣�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·����ͼ��ʾ��
A��ʯ���ѽ�������Ҫ�ɷ֣�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·����ͼ��ʾ�� CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��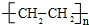 ��
��