��Ŀ����
3��ijʵ��С����H2O2�ֽ�Ϊ�����о�Ũ�ȡ���������Һ����ԶԷ�Ӧ���ʵ�Ӱ�죮�ڳ����°������·������ʵ�飮| ʵ���� | ��Ӧ�� | ���� |
| �� | 10mL2% H2O2��Һ | �� |
| �� | 10mL5% H2O2��Һ | �� |
| �� | 10mL5% H2O2��Һ | 1mL0.1mol•L-1FeCl3��Һ |
| �� | 10mL5% H2O2��Һ+����HCl��Һ | 1mL0.1mol•L-1FeCl3��Һ |
| �� | 10mL5% H2O2��Һ+����NaOH��Һ | 1mL0.1mol•L-1FeCl3��Һ |
��2��������5% H2O2��Һ��pHԼΪ6��H2O2�ĵ��뷽��ʽΪ⇒H2O2?H++HO2-��
��3��ʵ��ٺ͢ڵ�Ŀ����̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죮
��4��ʵ��ۡ��ܡ����У�������������������ʱ��仯�Ĺ�ϵ��ͼ��
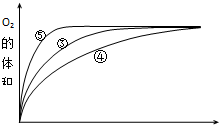
������ͼ�ܹ��ó���ʵ������Ǽ��Ի���������H2O2�ֽ�����ʣ����Ի����ܼ�СH2O2�ֽ�����ʣ�
���� ��1�������ı䷴Ӧ��;�������ͷ�Ӧ����Ļ�ܣ�
��2��pHԼΪ6����������������ӣ���Һ�����ԣ�
��3���Ƚ϶��գ�����ͬ��������ʵ��ٺ͢�H2O2��Һ��Ũ�Ȳ�ͬ��ʵ��Ŀ����̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죻
��4����ͼ��֪���ݵķ�Ӧ������ܵķ�Ӧ������С������ͼ����жϣ����Ի���������H2O2�ֽ�����ʣ����Ի����ܼ�СH2O2�ֽ�����ʣ�
��� �⣺��1�����ڴ����ı��˷�Ӧ��;�������ͷ�Ӧ����Ļ�ܣ��Ӷ��ӿ췴Ӧ���ʣ�
�ʴ�Ϊ�������˻�ܣ�
��2��������5%H2O2��Һ��pHԼΪ6����Һ�����ԣ�˵��˫��ˮ���Կ��������ᣬ�������뷽��ʽΪH2O2?H++HO2-��
�ʴ�Ϊ��H2O2?H++HO2-��
��3��ʵ���10mL2% H2O2��Һ��ʵ���10mL5% H2O2��Һ��������˫��ˮ��Ũ�Ȳ�ͬ������ʵ���Ŀ�������ʵ���Ŀ��Ϊ̽��Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬
�ʴ�Ϊ��̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죻
��4��ʵ��ۡ��ܡ����в�ͬ������Һ������ԣ���ͼ��֪���ݵķ�Ӧ������ܵķ�Ӧ������С�����ʵ�鷽����֪�����Ի���������H2O2�ֽ�����ʣ����Ի����ܼ�СH2O2�ֽ�����ʣ�
�ʴ�Ϊ�����Ի���������H2O2�ֽ�����ʣ����Ի����ܼ�СH2O2�ֽ�����ʣ�
���� ���⿼��Ӱ�컯ѧ��Ӧ���ʵ����أ��ϺõĿ���ѧ��ʵ����ơ����ݴ�����ͼ��������ۺ��������Ѷ��еȣ�ע����Ϣ�����ü��ɽ��

| A�� | Na2O2��CO2 | B�� | Na��O2 | C�� | NaOH��CO2 | D�� | NaAlO2��HNO3 |
| A�� | NH4Al��SO4��2��Һ�����NaOH��Һ��Ӧ��Al3++4OH-�TAlO2-+2H2O | |
| B�� | ICl�������ϡKOH��Һ�У�ICl+2OH-�TCl-+IO-+H2O | |
| C�� | �ö��Ե缫���CuSO4��Һ��2Cu2++4OH-$\frac{\underline{\;���\;}}{\;}$2Cu��+O2��+2H2O | |
| D�� | NaAlO2��Һ��AlO2-��ˮ�⣺AlO2-+2H2O�TAl��OH��3+OH- |
 ���ᣨH2C2O4����������Ȼ���ֲ���У���K1=5.4��10-2��K2=5.4��10-5�����л�ԭ�ԣ�����ˮ����Һ�����ԣ�Ϊ�ⶨijH2C2O4��Һ��Ũ�ȣ�ȡ����Һ����ƿ�У���������ϡH2SO4����Ũ��Ϊc mol/L KMnO4����Һ�ζ����ζ�ԭ��Ϊ��2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O
���ᣨH2C2O4����������Ȼ���ֲ���У���K1=5.4��10-2��K2=5.4��10-5�����л�ԭ�ԣ�����ˮ����Һ�����ԣ�Ϊ�ⶨijH2C2O4��Һ��Ũ�ȣ�ȡ����Һ����ƿ�У���������ϡH2SO4����Ũ��Ϊc mol/L KMnO4����Һ�ζ����ζ�ԭ��Ϊ��2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O��1���ζ�����ʹ��֮ǰ��������еIJ����Ǽ���Ƿ�©ˮ���ζ�ʱ��KMnO4��ҺӦװ����ʽ�ζ��ܣ����ʽ�ζ��ܡ���ʽ�ζ��ܡ����У��ﵽ�ζ��յ�ʱ������Ϊ�������һ��KMnO4��Һ����Һ����ɫ��Ϊdz�Ϻ�ɫ���Ұ���Ӳ���ɫ��
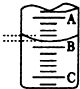
��2����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��C���Ŀ̶�Ϊ20���ζ�����Һ�����ӦΪ19.40mL����ʱ�ζ�����Һ����������30.60mL��������ڡ�С�ڻ���ڣ�
��3��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡH2C2O4��Һ�����ΪVmL������ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����KMnO4��Һ���/mL | 22.32 | 24.39 | 24.41 |
A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱKMnO4��Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
C����һ�εζ�ʢװ��Һ�ĵζ���װҺǰ������ˮ��ϴ����δ�ñ�Һ��ϴ��
D����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ��
E���μ�KMnO4��Һ���죬δ������տ�����Һ��ɫ������ֹͣ�ζ�
��4�������������ݣ�д��H2C2O4�����ʵ���Ũ�ȵı���ʽ�����뻯��C=$\frac{61c}{V}$mol/L��
��5������һ����Ƽ�ʵ��֤�����������ǿ��̼�ᣬʵ�������������ȡ������NaHCO3���Թ��У����������Һ�������ݲ�����

��1���ñ�����ζ����������������Һʱ����ͼ1����������ѡ����ѡ����ǡ����һ� �� ��
| ��ƿ����Һ | �ζ�������Һ | ѡ��ָʾ�� | ѡ�õζ��� | |
| A | �� | �� | ʯ�� | ���ң� |
| B | �� | �� | ���� | ���ף� |
| C | �� | �� | ��̪ | ���ף� |
| D | �� | �� | ʯ�� | ���ң� |
��2�����в����п���ʹ��������������Һ��Ũ��ֵƫ�͵�D
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B���ζ�ǰʢ���� ��������Һ����ƿ������ˮϴ����δ����
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ��������Ӷ���
��3��ijѧ������3��ʵ��ֱ��¼�й����������
| ������������ | 0.100mol/L�������� | ||
| �ζ����� | ��Һ�������mL�� | �ζ�ǰ�Ŀ̶ȣ�mL�� | �ζ���Ŀ̶ȣ�mL�� |
| ��һ�� | 25.00 | 1.68 | 26.89 |
| �ڶ��� | 25.00 | 0.00 | 27.91 |
| ������ | 25.00 | 0.12 | 25.01 |
��4����2ͼΪ����25mL NaOH��Һ����εμ�CH3COOH��Һ��������ҺpH�ı仯���ߣ���ش�
B����Һ�����ԣ����˾ݴ���Ϊ����B��ʱNaOH��CH3COOHǡ����ȫ��Ӧ�����ֿ����Ƿ���ȷ����ѡ��ǡ�����������ȷ�������ǡ����ȫ��Ӧ�ĵ�����AB���仹��BD�����ڣ�AB���䣮������ȷ�����ʲ��𣩣�
| A�� | ����������̼ͨ�롰ˮ�������У�C02+SiO32-+H20�TC032-+H2SiO3�� | |
| B�� | ��ˮ���� AlCl3 ��Һ�У�Al3++30H-�TAl��0H��3�� | |
| C�� | ���ܽ���NaOH��Һ�У�2Al+2OH-+6H2O�T2[Al��OH��4]-+3H2�� | |
| D�� | Al2O3���� NaOH ��Һ�У�Al2O3+2OH-+3H2O=2[Al��0H��4]- |
| A | C | |
| B |
��2��BԪ������������Ӧˮ����Ļ�ѧʽΪH2SO4���û�����Ϊ���ۣ�����ۡ������ӡ��������
��3��C��ԭ�ӽṹʾ��ͼΪ
 ��C�ĵ�����H2��Ӧ�Ļ�ѧ����ʽΪ��H2+F2=2HF
��C�ĵ�����H2��Ӧ�Ļ�ѧ����ʽΪ��H2+F2=2HF��4����Ԫ��A��C����Ԫ���γɵĻ������к��еĻ�ѧ�������������Ӽ������ۼ���
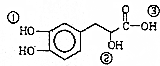
| A�� | ���л���ķ���ʽΪC9H10O5 | |
| B�� | ���л����ܷ������ۡ��ӳɡ���ȥ��������Ӧ | |
| C�� | 1mol���л��������Ժ�4molNaOH������Ӧ | |
| D�� | ���л�������Т١��ڡ���3��-OH��������ǿ������˳���Ǣۣ��٣��� |
| A�� | c��H+�� | B�� | c��H+��•c��OH-�� | C�� | $\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$ | D�� | $\frac{c��O{H}^{-}��}{c��{H}^{+}��}$ |