��Ŀ����
6��Ӱ�컯ѧ��Ӧ���ʵ����غܶ࣬ij������ȤС����ʵ��ķ�������̽����ʵ��һ����ͬѧ�о���ʵ�鱨�������
| ʵ �� �� �� | �� �� | �� �� |
| �ٷֱ�ȡ�������2mol/L�������Թ��У� �ڷֱ�Ͷ���С����״��ͬ��Cu��Fe��Mg�� | ��Ӧ������ Mg��Fe��Cu | ��Ӧ�������Խ���ã���Ӧ����Խ�죮 |
ʵ�������֪2KMnO4+5H2C2O4+3H2SO4�TK2SO4+2MnSO4+8H2O+10CO2�����ڸ������������Һ�Ͳ�����Һ��Ӧʱ�����ֿ�ʼһ��ʱ�䷴Ӧ���ʽ�������Һ��ɫ�����ԣ�������ͻȻ��ɫ����Ӧ�������Լӿ죮
��1���������ʵ������ijͬѧ��ΪKMnO4��H2C2O4��Ӧ�Ƿ��ȷ�Ӧ��������Һ�¶����ߣ���Ӧ���ʼӿ죮��Ӱ�컯ѧ��Ӧ���ʵ����ؿ�������뷴Ӧ�������Լӿ��ԭ���������ɵ��������д����ã�
��2������ʵ��֤����IJ��룬�����Ը��������Һ��������Һ�⣬����Ҫѡ����Լ����������B��ѡ���ţ���
A������� B�������� C��ˮ D���Ȼ��̣�
���� ʵ��һ������ʵ���еIJ�ͬ��ȷ��ʵ��Ŀ�ģ����Ա�ʵ��ʱֻ��һ��������ͬ����������������ȫ��ͬ��
ʵ�������1���������д����ã�
��2��Ҫ����֤�����ӵĴ����ã��ټ��������̼��ɣ�
��� �⣺ʵ��һ���÷�Ӧ���ἰ��Ũ����ͬ��������ͬ��������ʵ��Ŀ�����о���Ӧ�ﱾ�������ʶԷ�Ӧ���ʵ�Ӱ�죬���Ա�ʵ��ʱֻ��һ��������ͬ����������������ȫ��ͬ������Ҫ�ó���ȷ��ʵ����ۣ�������Ƶ�ʵ�������DZ����¶���ͬ��
�ʴ�Ϊ����Ӧ�ﱾ�������ʣ��¶ȣ�
ʵ�������1��KMnO4��H2C2O4��Ӧ���������̣��������д����ã����Բ��뻹�����Ǵ��������ã�
�ʴ�Ϊ�����ɵ��������д����ã�
��2��Ҫ����֤�����ӵĴ����ã������Ա�ʵ��ʱͬʱ���������̹۲췴Ӧ�����Ƿ�仯���ɣ����ܼ����Ȼ��̣������������Ӷ�ʵ���и��ţ�
�ʴ�Ϊ��B��
���� ���⿼����̽��Ӱ�컯ѧ��Ӧ���ʵ����أ�ע�����Ա�ʵ��ʱֻ�ܸı�һ����������������������ͬ���������ó���ȷ���ۣ�ע�������ӶԷ�Ӧ�Ĵ����ã�

��ϰ��ϵ�д�
�����Ŀ
4������ָ����Ӧ�����ӷ���ʽ��ȷ���ǣ�������
| A�� | NH4Al��SO4��2��Һ�����NaOH��Һ��Ӧ��Al3++4OH-�TAlO2-+2H2O | |
| B�� | ICl�������ϡKOH��Һ�У�ICl+2OH-�TCl-+IO-+H2O | |
| C�� | �ö��Ե缫���CuSO4��Һ��2Cu2++4OH-$\frac{\underline{\;���\;}}{\;}$2Cu��+O2��+2H2O | |
| D�� | NaAlO2��Һ��AlO2-��ˮ�⣺AlO2-+2H2O�TAl��OH��3+OH- |
11��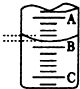 ���ᣨH2C2O4����������Ȼ���ֲ���У���K1=5.4��10-2��K2=5.4��10-5�����л�ԭ�ԣ�����ˮ����Һ�����ԣ�Ϊ�ⶨijH2C2O4��Һ��Ũ�ȣ�ȡ����Һ����ƿ�У���������ϡH2SO4����Ũ��Ϊc mol/L KMnO4����Һ�ζ����ζ�ԭ��Ϊ��2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O
���ᣨH2C2O4����������Ȼ���ֲ���У���K1=5.4��10-2��K2=5.4��10-5�����л�ԭ�ԣ�����ˮ����Һ�����ԣ�Ϊ�ⶨijH2C2O4��Һ��Ũ�ȣ�ȡ����Һ����ƿ�У���������ϡH2SO4����Ũ��Ϊc mol/L KMnO4����Һ�ζ����ζ�ԭ��Ϊ��2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O
��1���ζ�����ʹ��֮ǰ��������еIJ����Ǽ���Ƿ�©ˮ���ζ�ʱ��KMnO4��ҺӦװ����ʽ�ζ��ܣ����ʽ�ζ��ܡ���ʽ�ζ��ܡ����У��ﵽ�ζ��յ�ʱ������Ϊ�������һ��KMnO4��Һ����Һ����ɫ��Ϊdz�Ϻ�ɫ���Ұ���Ӳ���ɫ��
��2����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��C���Ŀ̶�Ϊ20���ζ�����Һ�����ӦΪ19.40mL����ʱ�ζ�����Һ����������30.60mL��������ڡ�С�ڻ���ڣ�
��3��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡH2C2O4��Һ�����ΪVmL������ʵ������¼���£�
���ϱ����Կ�������һ��ʵ���м�¼����KMnO4��Һ������������ں����Σ���ԭ�������AE
A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱKMnO4��Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
C����һ�εζ�ʢװ��Һ�ĵζ���װҺǰ������ˮ��ϴ����δ�ñ�Һ��ϴ��
D����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ��
E���μ�KMnO4��Һ���죬δ������տ�����Һ��ɫ������ֹͣ�ζ�
��4�������������ݣ�д��H2C2O4�����ʵ���Ũ�ȵı���ʽ�����뻯��C=$\frac{61c}{V}$mol/L��
��5������һ����Ƽ�ʵ��֤�����������ǿ��̼�ᣬʵ�������������ȡ������NaHCO3���Թ��У����������Һ�������ݲ�����
 ���ᣨH2C2O4����������Ȼ���ֲ���У���K1=5.4��10-2��K2=5.4��10-5�����л�ԭ�ԣ�����ˮ����Һ�����ԣ�Ϊ�ⶨijH2C2O4��Һ��Ũ�ȣ�ȡ����Һ����ƿ�У���������ϡH2SO4����Ũ��Ϊc mol/L KMnO4����Һ�ζ����ζ�ԭ��Ϊ��2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O
���ᣨH2C2O4����������Ȼ���ֲ���У���K1=5.4��10-2��K2=5.4��10-5�����л�ԭ�ԣ�����ˮ����Һ�����ԣ�Ϊ�ⶨijH2C2O4��Һ��Ũ�ȣ�ȡ����Һ����ƿ�У���������ϡH2SO4����Ũ��Ϊc mol/L KMnO4����Һ�ζ����ζ�ԭ��Ϊ��2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O��1���ζ�����ʹ��֮ǰ��������еIJ����Ǽ���Ƿ�©ˮ���ζ�ʱ��KMnO4��ҺӦװ����ʽ�ζ��ܣ����ʽ�ζ��ܡ���ʽ�ζ��ܡ����У��ﵽ�ζ��յ�ʱ������Ϊ�������һ��KMnO4��Һ����Һ����ɫ��Ϊdz�Ϻ�ɫ���Ұ���Ӳ���ɫ��
��2����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��C���Ŀ̶�Ϊ20���ζ�����Һ�����ӦΪ19.40mL����ʱ�ζ�����Һ����������30.60mL��������ڡ�С�ڻ���ڣ�
��3��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡH2C2O4��Һ�����ΪVmL������ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����KMnO4��Һ���/mL | 22.32 | 24.39 | 24.41 |
A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱKMnO4��Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
C����һ�εζ�ʢװ��Һ�ĵζ���װҺǰ������ˮ��ϴ����δ�ñ�Һ��ϴ��
D����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ��
E���μ�KMnO4��Һ���죬δ������տ�����Һ��ɫ������ֹͣ�ζ�
��4�������������ݣ�д��H2C2O4�����ʵ���Ũ�ȵı���ʽ�����뻯��C=$\frac{61c}{V}$mol/L��
��5������һ����Ƽ�ʵ��֤�����������ǿ��̼�ᣬʵ�������������ȡ������NaHCO3���Թ��У����������Һ�������ݲ�����
18��ijѧ��������֪���ʵ���Ũ�ȵĴ������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���ʵ���ָʾ��������д���пհ�

��1���ñ�����ζ����������������Һʱ����ͼ1����������ѡ����ѡ����ǡ����һ� �� ��
�ﵽ�ζ��յ�ʱ������Ϊ�ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ
��2�����в����п���ʹ��������������Һ��Ũ��ֵƫ�͵�D
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B���ζ�ǰʢ���� ��������Һ����ƿ������ˮϴ����δ����
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ��������Ӷ���
��3��ijѧ������3��ʵ��ֱ��¼�й����������
�����ϱ����ݼ��������������Һ�����ʵ���Ũ��Ϊ0.1002mol/L
��4����2ͼΪ����25mL NaOH��Һ����εμ�CH3COOH��Һ��������ҺpH�ı仯���ߣ���ش�
B����Һ�����ԣ����˾ݴ���Ϊ����B��ʱNaOH��CH3COOHǡ����ȫ��Ӧ�����ֿ����Ƿ���ȷ����ѡ��ǡ�����������ȷ�������ǡ����ȫ��Ӧ�ĵ�����AB���仹��BD�����ڣ�AB���䣮������ȷ�����ʲ��𣩣�

��1���ñ�����ζ����������������Һʱ����ͼ1����������ѡ����ѡ����ǡ����һ� �� ��
| ��ƿ����Һ | �ζ�������Һ | ѡ��ָʾ�� | ѡ�õζ��� | |
| A | �� | �� | ʯ�� | ���ң� |
| B | �� | �� | ���� | ���ף� |
| C | �� | �� | ��̪ | ���ף� |
| D | �� | �� | ʯ�� | ���ң� |
��2�����в����п���ʹ��������������Һ��Ũ��ֵƫ�͵�D
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B���ζ�ǰʢ���� ��������Һ����ƿ������ˮϴ����δ����
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ��������Ӷ���
��3��ijѧ������3��ʵ��ֱ��¼�й����������
| ������������ | 0.100mol/L�������� | ||
| �ζ����� | ��Һ�������mL�� | �ζ�ǰ�Ŀ̶ȣ�mL�� | �ζ���Ŀ̶ȣ�mL�� |
| ��һ�� | 25.00 | 1.68 | 26.89 |
| �ڶ��� | 25.00 | 0.00 | 27.91 |
| ������ | 25.00 | 0.12 | 25.01 |
��4����2ͼΪ����25mL NaOH��Һ����εμ�CH3COOH��Һ��������ҺpH�ı仯���ߣ���ش�
B����Һ�����ԣ����˾ݴ���Ϊ����B��ʱNaOH��CH3COOHǡ����ȫ��Ӧ�����ֿ����Ƿ���ȷ����ѡ��ǡ�����������ȷ�������ǡ����ȫ��Ӧ�ĵ�����AB���仹��BD�����ڣ�AB���䣮������ȷ�����ʲ��𣩣�
15��A��B��CΪ������Ԫ�أ������ڱ���������λ����ͼ��ʾ��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������Bԭ�Ӻ�������������������ȣ�
��1��д��A��CԪ�ص����Ƶ�����
��2��BԪ������������Ӧˮ����Ļ�ѧʽΪH2SO4���û�����Ϊ���ۣ�����ۡ������ӡ��������
��3��C��ԭ�ӽṹʾ��ͼΪ ��C�ĵ�����H2��Ӧ�Ļ�ѧ����ʽΪ��H2+F2=2HF
��C�ĵ�����H2��Ӧ�Ļ�ѧ����ʽΪ��H2+F2=2HF
��4����Ԫ��A��C����Ԫ���γɵĻ������к��еĻ�ѧ�������������Ӽ������ۼ���
| A | C | |
| B |
��2��BԪ������������Ӧˮ����Ļ�ѧʽΪH2SO4���û�����Ϊ���ۣ�����ۡ������ӡ��������
��3��C��ԭ�ӽṹʾ��ͼΪ
 ��C�ĵ�����H2��Ӧ�Ļ�ѧ����ʽΪ��H2+F2=2HF
��C�ĵ�����H2��Ӧ�Ļ�ѧ����ʽΪ��H2+F2=2HF��4����Ԫ��A��C����Ԫ���γɵĻ������к��еĻ�ѧ�������������Ӽ������ۼ���
16����NAΪ�����ӵ�������ֵ������˵����ȷ���ǣ�������
| A�� | ���³�ѹ�£�2.8g��ϩ���еĹ��õ��ӶԵ���ĿΪ0.5NA | |
| B�� | ��״���£���11.2LCl2ͨ������������������Һ���Ʊ�Ư��Һ��ת�Ƶĵ�����ΪNA | |
| C�� | VLamol/L���Ȼ�����Һ�У���Fe3+����ĿΪNA����Cl-����Ŀ����3NA | |
| D�� | T��ʱ��1LpH=6�Ĵ�ˮ�У����е�OH-����ĿΪ1.0��10-8NA |

 ���Ӿ۷�Ӧ��
���Ӿ۷�Ӧ��