��Ŀ����
8���л���F��һ������Ϳ�Ϲ̻�������������·�ߺϳɣ����ַ�Ӧ������ȥ����
��1��B�Ľṹ��ʽ��HOOC��CH2��4COOH����E�к��еĹ������������ǻ��Ͱ�����
��2����C��E�ϳ�F�Ļ�ѧ����ʽ��CH3OOC��CH2��4COOCH3+2HOCH2CH2NH2 $\stackrel{һ������}{��}$HOCH2CH2NHOC��CH2��4CONHCH2CH2OH+2CH3OH��
��3��ͬʱ�������������ı���ͬ���칹��Ľṹ��ʽ��
 ��
���ٺ���3��˫�����ں˴Ź�������ֻ��ʾ1�����շ壻�۲����ڼ�
��4����ϩ��ʵ���ҿ����Ҵ������л������ƣ�ͨ����ȥ��Ӧ���Ӧ�����ͣ��Ʊ���
��5������˵����ȷ����abd��
a��A ���ڱ����� b��D����ȩ�ķ���ʽ��ͬ
c��E���������ᷴӦ d��F���Է���������Ӧ��
���� ���������������£��������������ӳɷ�Ӧ����AΪ�����飬�����鱻������������BΪHOOC��CH2��4COOH���ڴ��������£�B�ͼ״�����������Ӧ����CΪCH3OOC��CH2��4COOCH3�����������������£���ϩ�������������ɻ������飬��������Ͱ�����Ӧ����EΪHOCH2CH2NH2��C��E��Ӧ����F������л���Ľṹ�����ʽ��
��� �⣺�����������������£��������������ӳɷ�Ӧ����AΪ�����飬�����鱻������������BΪHOOC��CH2��4COOH���ڴ��������£�B�ͼ״�����������Ӧ����CΪCH3OOC��CH2��4COOCH3�����������������£���ϩ�������������ɻ������飬��������Ͱ�����Ӧ����EΪHOCH2CH2NH2��C��E��Ӧ����F��
��1��B��1��6-�����ᣬ��ṹ��ʽΪ��HOOC��CH2��4COOH������E�Ľṹ��ʽ֪��E�к����ǻ��Ͱ�����
�ʴ�Ϊ��HOOC��CH2��4COOH���ǻ��Ͱ�����
��2��C��E����ȡ����Ӧ����F����Ӧ����ʽΪ��CH3OOC��CH2��4COOCH3+2HOCH2CH2NH2 $\stackrel{һ������}{��}$HOCH2CH2NHOC��CH2��4CONHCH2CH2OH+2CH3OH��
�ʴ�Ϊ��CH3OOC��CH2��4COOCH3+2HOCH2CH2NH2 $\stackrel{һ������}{��}$HOCH2CH2NHOC��CH2��4CONHCH2CH2OH+2CH3OH��
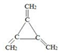
��3���߱��ٺ���3��˫�����ں˴Ź�������ֻ��ʾ1�����շ�����л�����ֻ��һ�����͵���ԭ�ӡ��۲����ڼ��ı���ͬ���칹��Ľṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��4����ϩ��ʵ���ҿ����Ҵ�ͨ����ȥ��Ӧ��ȡ��
�ʴ�Ϊ���Ҵ�����ȥ��Ӧ��
��5��a��AΪ�����飬��������ֻ���ڹ��۵������������ڱ�����������ȷ��
b��������������ȩ�ķ���ʽ����C2H4O�����Է���ʽ��ͬ������ȷ��
c��E�к��а����������������ᷴӦ���ʴ���
d��F�к����ǻ������Կ��Է���������Ӧ������ȷ��
��ѡabd��
���� �����漰�л�������֮���ת����ϵ�������ż����ʡ��л���Ӧ���͡���������ͬ���칹�����д�����֪ʶ����ȷ�л���Ĺ����ż��������ǽⱾ��ؼ����Ѷ��еȣ�

 �żӾ���ϵ�д�
�żӾ���ϵ�д�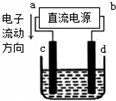
| A�� | a������b���� | B�� | a������b���� | ||
| C�� | �������У�������Ũ�Ȳ��� | D�� | �������У�d�缫������� |
| A�� | ��ȫ����56g Fe��Ҫ��������33.6 L | |
| B�� | 7.8g Na2O2����100mlˮ��ת�Ƶ�����Ϊ0.1NA | |
| C�� | ��100mL 1mol/L��NaHSO3��Һ�м�������������������Ӧ������������0.4mol | |
| D�� | �������ȷ�Ӧ��������ԭ�õ�16.8g�����ʣ���Ӧ��Fe�õ���0.9NA���� |
| A�� | AlCl3��Һ�м��������ˮ��Al3++4NH3•H2O�TAlO${\;}_{2}^{-}$+4NH${\;}_{4}^{+}$+2H2O | |
| B�� | �ں����ҵĽ���Һ������I-���еμ�H2O2�õ�I2��2I-+H2O2+2H+�TI2+O2��+2H2O | |
| C�� | ������������Һ�еμ����������Һ��PH=7��Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O | |
| D�� | ��̼������Һ���ݹ�¯ˮ����Ca2++CO32-�TCaCO3�� |
| A�� | �������ڶ�ͯ���ݵ����� | |
| B�� | �������ڹ����������ͷ��� | |
| C�� | �������ڻ�����Ca10��PO4��6��OH��2������ | |
| D�� | ��ʹ��ͯ������ |