��Ŀ����
8����֪��ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ���������������ԭ������ش��������⣺��1�������£�Ũ�Ⱦ�Ϊ0.1mol•L-1������������Һ��pH���±���
| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN |
| pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.0 |
�ڸ��ݱ��������жϣ�Ũ�Ⱦ�Ϊ0.01mol•L-1�������������ʵ���Һ�У�������ǿ����C��
�����ţ���ͬ��������Һ�ֱ�ϡ��100����pH�仯��С����A
A��HCN B��HClO C��H2CO3 D��CH3COOH
�۸����ϱ����ݣ������0.1mol•L-1��NaCN��Һ��ˮ����������������ӵ�Ũ��10-3mol/L
��Ҫ������ˮ��HClO��Ũ�ȣ�������ˮ�м���������̼������Һ������ƽ��ԭ��ԭ��HClO��̼���Ʋ���Ӧ���������̼���Ʒ�Ӧ���ɶ�����̼�����Ե�����ˮ��HClOŨ������2Cl2+CO32-+H2O=CO2��+2Cl-+2HClO��д���ӷ���ʽ���ñ�Ҫ������˵��������0�֣���
��2����0.1mol•L-1��NaClO��Һ�У�д������Ũ�ȵĴ�С��ϵc��Na+����c��ClO-����c��OH-����c��H+����
���� ��1�������������ˮ��̶�Խ����ͬŨ�ȵ�������Һ��pHԽ����������ӽ����������Խ��
���������Խǿ����ĵ���̶�Խ�����������ˮ��̶�ԽС����ͬŨ�ȵ�������Һ��pHԽС����ˮϡ�ʹٽ�������룬��ͬŨ�ȵIJ�ͬ��ϡ����ͬ�ı������������Խ�������PH�仯ԽС��
��0.1mol•L-1��NaCN��ҺpH=11������Һ���������������Ӿ�������ˮ�ĵ��룬�ݴ˷�����
��HClO��̼���Ʋ���Ӧ�������̼���Ʒ�Ӧ���ɶ�����̼��
��2��NaClO��ǿ�������Σ�ˮ���Լ��ԣ�
��� �⣺��1�������������ˮ��̶�Խ����ͬŨ�ȵ�������Һ��pHԽ����������ӽ����������Խ������ҺpH֪��CO32-��ˮ��̶������CO32-�������������ǿ���ʴ�Ϊ��CO32-��
���������Խǿ����ĵ���̶�Խ�����������ˮ��̶�ԽС����ͬŨ�ȵ�������Һ��pHԽС�����ݱ�������֪��������ǿ������C����������������HCN����ˮϡ�ʹٽ�������룬��ͬŨ�ȵIJ�ͬ��ϡ����ͬ�ı������������Խ�������PH�仯ԽС����������������HCN����pH�仯��С����HCN���ʴ�Ϊ��C��A��
��0.1mol•L-1��NaCN��ҺpH=11������Һ���������������Ӿ�������ˮ�ĵ��룬����Һ��ˮ���������������Ũ��Ϊ10-3mol/L���ʴ�Ϊ��10-3mol/L��
��HClO��̼���Ʋ���Ӧ�������̼���Ʒ�Ӧ���ɶ�����̼�����Ե�����ˮ��HClOŨ���������ӷ���ʽΪ2Cl2+CO32-+H2O=CO2��+2Cl-+2HClO��
�ʴ�Ϊ��HClO��̼���Ʋ���Ӧ�������̼���Ʒ�Ӧ���ɶ�����̼�����Ե�����ˮ��HClOŨ������2Cl2+CO32-+H2O=CO2��+2Cl-+2HClO��
��2��NaClO��ǿ�������Σ�ˮ���Լ��ԣ�����c��OH-����c��H+��������Һ�ʵ����ԣ�����c��Na+����c��ClO-�������У�c��Na+����c��ClO-����c��OH-����c��H+�����ʴ�Ϊ��c��Na+����c��ClO-����c��OH-����c��H+����
���� ���⿼���������ˮ��̶������ǿ���Ĺ�ϵ������Һ����������Ũ�ȵļ��������Ũ�ȴ�С�Ƚϵ����⣬�ѶȲ���Ӧע�������Խ��������Խˮ�⣮

| A�� | ����ͼ��� | B�� | CH3COOH��C3H6O2 | C�� |  �� ��  | D�� | HCHO��CH3COOH |
| A�� | ����ȼ�ϵ�صĵ������KOH��Һʱ���为���缫��ӦΪ2H2-4e-+4OH-�T4H2O | |
| B�� | ȼ�ϵ�ظ���ȼ��ʧ���ӣ�������ԭ��Ӧ | |
| C�� | ���Լ���ȼ�ϵ�ص�������ӦΪO2+2H2O+4e-�T4OH- | |
| D�� | �������⣬�����е�����Ҳ������������ |
| A�� | �٢� | B�� | �ۢ� | C�� | �٢� | D�� | �٢ڢ� |
 �������Ǵӷ������з������һ���л��ᣬ���кܺõ�ɱ���������������估ǿ�����塢��ǿ�����������ã���ṹ��ͼ��ʾ���й��������˵������ȷ���ǣ�������
�������Ǵӷ������з������һ���л��ᣬ���кܺõ�ɱ���������������估ǿ�����塢��ǿ�����������ã���ṹ��ͼ��ʾ���й��������˵������ȷ���ǣ�������| A�� | ����ʽΪC10H18O3 | |
| B�� | ��ʹ������Ȼ�̼��Һ��ɫ | |
| C�� | һ���������ܷ���ȡ����Ӧ��������Ӧ | |
| D�� | 1mol������������к�2molNaOH |
| A�� | dԪ�صķǽ�������ǿ | |
| B�� | ���Ǿ��������ֻ��������ϵ������� | |
| C�� | a����c��dԪ�طֱ��γ����ӻ����� | |
| D�� | b��c��d�ڻ�ѧ��Ӧ�о��õ����� |
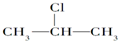 +NaOH$��_{��}^{��}$CH3-CH=CH2+NaCl+H2O
+NaOH$��_{��}^{��}$CH3-CH=CH2+NaCl+H2O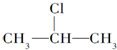 $��_{-NaCl��-H_{2}O}^{NaOH��������}$CH3-CH=CH2
$��_{-NaCl��-H_{2}O}^{NaOH��������}$CH3-CH=CH2
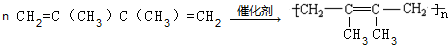 ��
��

 $��_{��}^{Ũ����}$
$��_{��}^{Ũ����}$ +H2O��
+H2O��
 ��
�� ��
�� Ϊԭ�Ϻϳ���Ҫ�Ļ�����Ʒ
Ϊԭ�Ϻϳ���Ҫ�Ļ�����Ʒ ��·������ͼ�����Լ���ѡ��
��·������ͼ�����Լ���ѡ��
 ��
��