��Ŀ����
14�������£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±�����ش�| ʵ���� | HA���ʵ���Ũ��/��mol•L-1�� | NaOH���ʵ���Ũ��/��mol•L-1�� | �����Һ��pH |
| �� | c | 0.2 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=9 |
��2����������ʵ����������HA�����ᣨѡ�ǿ�������������û����Һ������Ũ���ɴ�С��˳����c��Na+����c��A-����c��OH-����c��H+����
��3������ʵ�����û����Һ��������ʽ�ľ�ȷ�����c��OH-��-c��HA��=10-9mol/L��
���� ��1����HAΪǿ����c����0.2����HAΪ���ᣬ��c����0.2������c��һ��Ϊ0.2��
��2��Ϊ��Ũ��NaA��HA�����Һ����Ϻ���ҺpH��7����HAΪ���ᣬHA�ĵ���̶�С��NaA��ˮ��̶ȣ�
��3��ǡ�÷�Ӧ�õ�NaA��Һ�����������������غ�c��OH-��-c��HA��=c��H+����
��� �⣺��1���������������ʵ�����������������������pH=7����HAΪǿ�ᣬ��c����0.2����HAΪ���ᣬ��c����0.2������c��һ��Ϊ0.2��
�ʴ�Ϊ����
��2��Ϊ��Ũ��NaA��HA�����Һ����Ϻ���ҺpH��7����HAΪ���ᣬHA�ĵ���̶�С��NaA��ˮ��̶ȣ���Һ�У�c��Na+����c��A-����c��OH-����c��H+����
�ʴ�Ϊ����c��Na+����c��A-����c��OH-����c��H+����
��3��ǡ�÷�Ӧ�õ�NaA��Һ�����������������غ�c��OH-��-c��HA��=c��H+��=10-9mol/L��
�ʴ�Ϊ��10-9��
���� ���⿼���Ϊ�ۺϣ��漰������ˮ�⡢��ҺpH���㡢����Ũ�ȴ�С�Ƚ�֪ʶ����һ�����ۺ��ԣ�������ѧ���ķ��������ͼ��������Ŀ��飬��������ⷽʽΪ�߿�������ȵ㣬�����Ѷ����У�

��ϰ��ϵ�д�
�����Ŀ
1��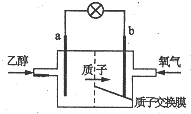 �����Ҵ�ȼ�ϵ�رȼ״�ȼ�ϵ�ص�Ч�ʸ߳�32�����Ҹ���ȫ����ṹ��ͼ��ʾ�����õ����������ܼ�����200������ʱ���磬��֪����ܷ�ӦʽΪ��C2H5OH+3O2=2CO2+3H2O������˵������ȷ���ǣ�������
�����Ҵ�ȼ�ϵ�رȼ״�ȼ�ϵ�ص�Ч�ʸ߳�32�����Ҹ���ȫ����ṹ��ͼ��ʾ�����õ����������ܼ�����200������ʱ���磬��֪����ܷ�ӦʽΪ��C2H5OH+3O2=2CO2+3H2O������˵������ȷ���ǣ�������
 �����Ҵ�ȼ�ϵ�رȼ״�ȼ�ϵ�ص�Ч�ʸ߳�32�����Ҹ���ȫ����ṹ��ͼ��ʾ�����õ����������ܼ�����200������ʱ���磬��֪����ܷ�ӦʽΪ��C2H5OH+3O2=2CO2+3H2O������˵������ȷ���ǣ�������
�����Ҵ�ȼ�ϵ�رȼ״�ȼ�ϵ�ص�Ч�ʸ߳�32�����Ҹ���ȫ����ṹ��ͼ��ʾ�����õ����������ܼ�����200������ʱ���磬��֪����ܷ�ӦʽΪ��C2H5OH+3O2=2CO2+3H2O������˵������ȷ���ǣ�������| A�� | ��ع���ʱ������b���ص��߾����ݵ�a�� | |
| B�� | a��Ϊ��صĸ������õ缫����������Ӧ | |
| C�� | ��������ĵ缫��ӦʽΪO2+2H2O+4e-=4OH- | |
| D�� | ��ع���ʱ��23g�Ҵ�������ת�Ƶ���ʽΪ6mol |
2����֪�Ȼ�����Һ��⻯����Һ���ʱ������Ӧ��2Fe3+��aq��+2I-��aq��=2Fe2+��aq��+I2��s�����ֽ�1 L 0.25mol•L-1�Ȼ�����Һ��4 L 0.10 mol•L-�⻯����Һ��Ϻ��ڵ�2minʱ����û����Һ��c��I-��=0.04 mo1•L-��������˵����ȷ���ǣ�������
| A�� | ��2minʱ��c��Fe2+��=0.01 mol•L-1 | |
| B�� | �����Һ�У�c��K+��=0.01 mol•L-1 | |
| C�� | 0��2min�ڣ�v��I-��=0.01 mol•L-1•min-1 | |
| D�� | ��2minʱ��c��Fe3+��=0.01 mol•L-1 |
2��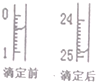 ����0.1000mol•L-1NaOH��Һͨ���к͵ζ��ⶨ������Һ�����ʵ���Ũ�ȣ��ش��������⣮
����0.1000mol•L-1NaOH��Һͨ���к͵ζ��ⶨ������Һ�����ʵ���Ũ�ȣ��ش��������⣮
��1��0.1000mol•L-1NaOH��Һ����ˮ�������c��OH��-=1��10-13mol•L-1
��2���ζ�ʱ����ʽ�����ʽ����ʽ�����ζ��ܽ�����Һ������ƿ�У�
��3�����ζ�ʱѡ�õ�ָʾ���Ƿ�̪�����յ�������ǵ������һ��NaOH��Һ����Һ����ɫ��Ϊ�ۺ�ɫ���Ұ�����ڲ���ɫ
��4���к͵ζ��й����ݼ�¼���±�����ͼ��ʾ�±��е�1�εζ�ʱ50mL�ζ�����ǰ��Һ���λ��
�����z=24.50mL
��5�������������ݣ�������Һ�����ʵ���Ũ��c=0.1020mol•L-1��������λ��Ч���֣�
��6�����в�����ʹ�ⶨ���ƫ�ߵ���ad
a��ϴ�Ӽ�ʽ�ζ���ʱδ��װ��Һ��ϴ
b��ϴ��ʱ��ƿֻ������ˮϴ�����δ��ϴ
c�������ñ�Һ���ʱ���ζ�ǰ���ӣ��ζ�������
d���ζ�ʱ��Һ��С�ĵ��뵽��ƿ���森
 ����0.1000mol•L-1NaOH��Һͨ���к͵ζ��ⶨ������Һ�����ʵ���Ũ�ȣ��ش��������⣮
����0.1000mol•L-1NaOH��Һͨ���к͵ζ��ⶨ������Һ�����ʵ���Ũ�ȣ��ش��������⣮��1��0.1000mol•L-1NaOH��Һ����ˮ�������c��OH��-=1��10-13mol•L-1
��2���ζ�ʱ����ʽ�����ʽ����ʽ�����ζ��ܽ�����Һ������ƿ�У�
��3�����ζ�ʱѡ�õ�ָʾ���Ƿ�̪�����յ�������ǵ������һ��NaOH��Һ����Һ����ɫ��Ϊ�ۺ�ɫ���Ұ�����ڲ���ɫ
��4���к͵ζ��й����ݼ�¼���±�����ͼ��ʾ�±��е�1�εζ�ʱ50mL�ζ�����ǰ��Һ���λ��
| �ζ���� | ����Һ�����mL�� | ������NaOH��Һ�������mL�� | ||
| �ζ�ǰ | �ζ��� | ���ĵ���� | ||
| 1 | 25.00 | x | y | z |
| 2 | 25.00 | 4.00 | 29.60 | 25.60 |
| 3 | 25.00 | 0.60 | 26.00 | 25.40 |
��5�������������ݣ�������Һ�����ʵ���Ũ��c=0.1020mol•L-1��������λ��Ч���֣�
��6�����в�����ʹ�ⶨ���ƫ�ߵ���ad
a��ϴ�Ӽ�ʽ�ζ���ʱδ��װ��Һ��ϴ
b��ϴ��ʱ��ƿֻ������ˮϴ�����δ��ϴ
c�������ñ�Һ���ʱ���ζ�ǰ���ӣ��ζ�������
d���ζ�ʱ��Һ��С�ĵ��뵽��ƿ���森
9�������£����и���������ָ����Һ��һ���ܴ���������ǣ�������
| A�� | 0.1mol•L-1NaHCO3��Һ��H+��Al3+��Cl-��CH3COO- | |
| B�� | 0.1mol•L-1CuCl2��Һ��K+��NH4+��OH-��S2- | |
| C�� | 0.1mol•L-1FeCl2��Һ��Na+��NH4+��I-��SO42- | |
| D�� | $\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=1��1013����Һ��Fe3+��Na+��SO32����NO3- |
19��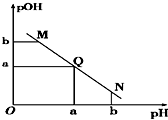 ij�¶��£���һ�����1mol/L������Һ����μ����Ũ�ȵ�NaOH��Һ����Һ��pOH��pOH=-lg c[OH-]����pH�ı仯��ϵ��ͼ��ʾ��������
ij�¶��£���һ�����1mol/L������Һ����μ����Ũ�ȵ�NaOH��Һ����Һ��pOH��pOH=-lg c[OH-]����pH�ı仯��ϵ��ͼ��ʾ��������
 ij�¶��£���һ�����1mol/L������Һ����μ����Ũ�ȵ�NaOH��Һ����Һ��pOH��pOH=-lg c[OH-]����pH�ı仯��ϵ��ͼ��ʾ��������
ij�¶��£���һ�����1mol/L������Һ����μ����Ũ�ȵ�NaOH��Һ����Һ��pOH��pOH=-lg c[OH-]����pH�ı仯��ϵ��ͼ��ʾ��������| A�� | M����ʾ��Һ��������ǿ��Q�� | |
| B�� | N����ʾ��Һ��c��CH3COO-����c��Na+�� | |
| C�� | M���N����ʾ��Һ��ˮ�ĵ���̶Ȳ���ͬ | |
| D�� | Q������NaOH��Һ�����С�ڴ�����Һ����� |
6������ʱ����Ũ�Ⱥ�����ֱ�ΪC1��V1��NaOH��Һ��C2��V2��CH3COOH��Һ���ϣ����й��ڸû����Һ������������ǣ�������
| A�� | ��pH��7ʱ����һ����C1V1��C2V2 | |
| B�� | ��pH��7ʱ�������Һ�п�����c��Na+����c��H+�� | |
| C�� | ��pH=7ʱ����V1=V2����һ����C2=C1 | |
| D�� | �� V1=V2��C1=C2����c��CH3COO-��+c��CH3COOH��=c��Na+�� |
3������������ȷ���ǣ�������
| A�� | ���к��ȵIJⶨʵ���У�Ӧ����Һ����������Һ�У�ʹ��Ӧ��ֽ��� | |
| B�� | ��NaOH����Һ�ζ�������Һ������ʱ����������ȫ�к� | |
| C�� | ��Na2S2O3��Һ��ϡ�����ϣ���ͨ���۲�������ݵĿ������жϻ�ѧ��Ӧ���� | |
| D�� | ���������Թ��У�����2mLˮ��3��ϡ�����1��K3[Fe��CN��6]��Һ������������Χ������ɫ���� |
4���������ϣ���ˮ����������ɫ��ζ�Ľᾧ�壬�ڿ����пɱ��绯����ȼ������ˮ���������ʯ�Ҽ��ȿ����Ƶ�X���壮������Ӧ��ʵ�飺
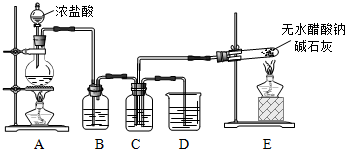
��1��װ��E�з�Ӧ����ʽ��CH3COONa+NaOH$��_{��}^{CaO}$Na2CO3+X����X�Ļ�ѧʽ��CH4��
��2����C���ռ�����X��Cl2Ϊ1��1�����ϣ�Ȼ���ڹ����·�Ӧ��
��Bװ�õ������dz�ȥCl2�к��е�HCl��
��Cװ����ʢ���Լ��DZ���ʳ��ˮ�����պ����ò�����CH3Cl��CH2Cl2��CHCl3��CCl4��HCl��
��3�����һ��ʵ�鷽������֤������������һ��������պ�ȡC��D��Һ����������ֱ������֧�Թ��У��ٷֱ�μ�2��ʯ����Һ��C����Һ��ɫ�������pH��ֽ�ⶨC��D����Һ��pH��
��4��ijѧ��Ϊ�˲ⶨX����ɽ���ʵ��̽����ѡ��������������
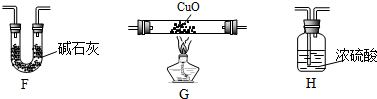
�ٸ�ͬѧ�������ӵĺ���˳����E��G��H��F��
�ڸ�ͬѧ�ⶨ����������ǣ�
�����Ƿ���ȫ��˵��ԭ�������Dz���ȫ����Ϊֻ��ȷ���л����е�̼��Ԫ�غ���������ȷ���Ƿ�����Ԫ�أ�Ӧ��ȡCװ��ʵ��ǰ���������
�òⶨ�������Ƿ���ƫ�����ƫ���˵��ԭ��F�����յ���CO2��CO2����33.6g-22.5g=11.1g��n��C��=$\frac{11.1g}{44g/mol}$=0.252mol��H�����յ���ˮ������H2O����51.4g-42.4g=9.0g��n��H��=$\frac{9.0g}{18g/mol}$��2=1.0mol��
��n��C����n��H����1��4��ԭ����Fװ�ú���������Ӵ��������е�ˮ������CO2������Bװ�ã���ɺ�̼��ƫ��

��1��װ��E�з�Ӧ����ʽ��CH3COONa+NaOH$��_{��}^{CaO}$Na2CO3+X����X�Ļ�ѧʽ��CH4��
��2����C���ռ�����X��Cl2Ϊ1��1�����ϣ�Ȼ���ڹ����·�Ӧ��
��Bװ�õ������dz�ȥCl2�к��е�HCl��
��Cװ����ʢ���Լ��DZ���ʳ��ˮ�����պ����ò�����CH3Cl��CH2Cl2��CHCl3��CCl4��HCl��
��3�����һ��ʵ�鷽������֤������������һ��������պ�ȡC��D��Һ����������ֱ������֧�Թ��У��ٷֱ�μ�2��ʯ����Һ��C����Һ��ɫ�������pH��ֽ�ⶨC��D����Һ��pH��
��4��ijѧ��Ϊ�˲ⶨX����ɽ���ʵ��̽����ѡ��������������

�ٸ�ͬѧ�������ӵĺ���˳����E��G��H��F��
�ڸ�ͬѧ�ⶨ����������ǣ�
| װ�� | ʵ��ǰ������/g | ʵ��������/g |
| F | 22.5 | 33.6 |
| H | 42.4 | 51.4 |
�òⶨ�������Ƿ���ƫ�����ƫ���˵��ԭ��F�����յ���CO2��CO2����33.6g-22.5g=11.1g��n��C��=$\frac{11.1g}{44g/mol}$=0.252mol��H�����յ���ˮ������H2O����51.4g-42.4g=9.0g��n��H��=$\frac{9.0g}{18g/mol}$��2=1.0mol��
��n��C����n��H����1��4��ԭ����Fװ�ú���������Ӵ��������е�ˮ������CO2������Bװ�ã���ɺ�̼��ƫ��