��Ŀ����
4���������ϣ���ˮ����������ɫ��ζ�Ľᾧ�壬�ڿ����пɱ��绯����ȼ������ˮ���������ʯ�Ҽ��ȿ����Ƶ�X���壮������Ӧ��ʵ�飺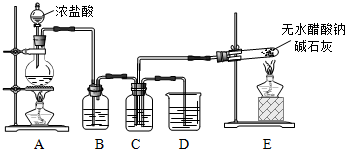
��1��װ��E�з�Ӧ����ʽ��CH3COONa+NaOH$��_{��}^{CaO}$Na2CO3+X����X�Ļ�ѧʽ��CH4��
��2����C���ռ�����X��Cl2Ϊ1��1�����ϣ�Ȼ���ڹ����·�Ӧ��
��Bװ�õ������dz�ȥCl2�к��е�HCl��
��Cװ����ʢ���Լ��DZ���ʳ��ˮ�����պ����ò�����CH3Cl��CH2Cl2��CHCl3��CCl4��HCl��
��3�����һ��ʵ�鷽������֤������������һ��������պ�ȡC��D��Һ����������ֱ������֧�Թ��У��ٷֱ�μ�2��ʯ����Һ��C����Һ��ɫ�������pH��ֽ�ⶨC��D����Һ��pH��
��4��ijѧ��Ϊ�˲ⶨX����ɽ���ʵ��̽����ѡ��������������
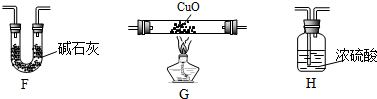
�ٸ�ͬѧ�������ӵĺ���˳����E��G��H��F��
�ڸ�ͬѧ�ⶨ����������ǣ�
| װ�� | ʵ��ǰ������/g | ʵ��������/g |
| F | 22.5 | 33.6 |
| H | 42.4 | 51.4 |
�òⶨ�������Ƿ���ƫ�����ƫ���˵��ԭ��F�����յ���CO2��CO2����33.6g-22.5g=11.1g��n��C��=$\frac{11.1g}{44g/mol}$=0.252mol��H�����յ���ˮ������H2O����51.4g-42.4g=9.0g��n��H��=$\frac{9.0g}{18g/mol}$��2=1.0mol��
��n��C����n��H����1��4��ԭ����Fװ�ú���������Ӵ��������е�ˮ������CO2������Bװ�ã���ɺ�̼��ƫ��
���� ��1������ԭ���غ�õ�����Ļ�ѧʽ��
��2����װ��A����������װ�ã����ɵ������к��Ȼ������壬��Ҫͨ������ʳ��ˮ��ȥ��װ��E�Ǽ������巢��װ�ã������ͼ��鰴��1��1�����Ϲ��շ���ȡ����Ӧ�����ɵ�����ͨ��װ��D���գ�
��װ��C���DZ���ʳ��ˮ�����������ܽ⣬��Ӧ��������Ӧ���õ�����ȡ��������Ȼ������壻
��3���������е�����Ϊ�Ȼ��⣬����ˮ�γ����ᣬ�������������ʵ����飻
��4����Ϊ�˲ⶨXΪCH4����ɣ�����װ��E�Ʊ����飬�õ�����ͨ��װ��G�е�����ͭ��
��ʵ������ֻ�ܼ���̼����Ԫ�����ʵ���֮�ȣ�����ȷ���Ƿ���Ԫ�أ�F�����յ���CO2��CO2 ����33.6g-22.5g=11.1g��n��C��=$\frac{11.1g}{44g/mol}$=0.252mol��H �����յ���ˮ������H2O����5 1.4g-42.4g=9.0g��n��H��=$\frac{9.0g}{18g/mol}$��2=1.0mol����n��C����n��H����1��4����ʯ�һ����տ����ж�����̼��ˮ����������������
��� �⣺��1��װ��E�з�Ӧ����ʽ��CH3COONa+NaOH$��_{��}^{CaO}$Na2CO3+X����X�Ļ�ѧʽ��CH4��
�ʴ�Ϊ��CH4��
��2����Bװ�õ������dz�ȥCl2�к��е�HCl����ֹ���Ų���ļ��飬
�ʴ�Ϊ����ȥCl2�к��е�HCl��
��Cװ����ʢ�б���ʳ��ˮ�����������ܽ⣬ʹ�����������Ͼ��ȣ����պ������ͼ��鷢��ȡ����Ӧ����һ�ȼ��顢���ȼ��顢���ȼ��顢���ȼ�����Ȼ��⣬�����Ȼ�������ˮ��Һ����������ǿ��������Ļ�ѧʽΪ��CH3Cl��CH2Cl2��CHCl3��CCl4��HCl��
�ʴ�Ϊ������ʳ��ˮ��CH3Cl��CH2Cl2��CHCl3��CCl4��HCl��
��3���������е�����Ϊ�Ȼ��⣬����ˮ�γ����ᣬ���պ�ȡC��D��Һ����������ֱ������֧�Թ��У��ٷֱ�μ�2��ʯ����Һ��C����Һ��ɫ�������pH ��ֽ�ⶨC��D����Һ��pH �ȣ�
�ʴ�Ϊ�����պ�ȡC��D��Һ����������ֱ������֧�Թ��У��ٷֱ�μ�2��ʯ����Һ��C����Һ��ɫ�������pH ��ֽ�ⶨC��D����Һ��pH �ȣ�
��4���ٲⶨ������ɣ��Ƚ������������ɶ�����̼��ˮ����ʯ��ͬʱ���ն�����̼��ˮ��������Ũ��������ˮ�����ü�ʯ�����ն�����̼�����ɵļ���ͨ�����ȵ�����ͭ��������ˮ�Ͷ�����̼��װ������˳��ΪEGHF��
�ʴ�Ϊ��G��H��F��
��ʵ������ֻ�ܼ���̼����Ԫ�����ʵ���֮�ȣ�����ȷ���Ƿ���Ԫ�أ�����Ӧ���������غ�����ж��Ƿ���Ԫ�أ���Ҫ��ȡC װ��ʵ��ǰ���������
F�����յ���CO2��CO2 ����33.6g-22.5g=11.1g��n��C��=$\frac{11.1g}{44g/mol}$=0.252mol��H �����յ���ˮ������H2O����5 1.4g-42.4g=9.0g��n��H��=$\frac{9.0g}{18g/mol}$��2=1.0mol��
��n��C����n��H����1��4�����Լ�������֪�������ԭ����F װ�ú���������Ӵ��������е�ˮ������CO2 ������B װ�ã���ɺ�̼��ƫ��
�ʴ�Ϊ�������Dz���ȫ����Ϊֻ��ȷ���л����е�̼��Ԫ�غ���������ȷ���Ƿ�����Ԫ�أ�Ӧ��ȡC װ��ʵ��ǰ���������F�����յ���CO2��CO2 ����33.6g-22.5g=11.1g��n��C��=$\frac{11.1g}{44g/mol}$=0.252mol��H �����յ���ˮ������H2O����5 1.4g-42.4g=9.0g��n��H��=$\frac{9.0g}{18g/mol}$��2=1.0mol����n��C����n��H����1��4��ԭ����F װ�ú���������Ӵ��������е�ˮ������CO2������B װ�ã���ɺ�̼��ƫ��
���� ���⿼������������ʵ�������ʵ�鷽����ƣ���Ҫ��������ɵ�ʵ���÷�����װ�����ӵ�֪ʶ�㣬����ʵ����������������ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| ʵ���� | HA���ʵ���Ũ��/��mol•L-1�� | NaOH���ʵ���Ũ��/��mol•L-1�� | �����Һ��pH |
| �� | c | 0.2 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=9 |
��2����������ʵ����������HA�����ᣨѡ�ǿ�������������û����Һ������Ũ���ɴ�С��˳����c��Na+����c��A-����c��OH-����c��H+����
��3������ʵ�����û����Һ��������ʽ�ľ�ȷ�����c��OH-��-c��HA��=10-9mol/L��
| A�� | ��Һ��Na+Ũ��������O2�ų� | B�� | ��ҺpHֵ���䣬��H2�ų� | ||
| C�� | ��Һ��Na+��Ŀ���٣���O2�ų� | D�� | ��Һ��pHֵ������O2�ų� |
| A�� | Ag+ | B�� | Cu2+ | C�� | Na+ | D�� | Hg2+ |
 ���ᡢ�����̼���ǻ�ѧʵ����о��г��õļ����ᣮ�����£�Ka��CH3COOH��=1.7��10-5 mol/L��H2CO3�ĵ��볣��Ka1=4.2��10-7mol•L-1��Ka2=5.6��10-11mol•L-1
���ᡢ�����̼���ǻ�ѧʵ����о��г��õļ����ᣮ�����£�Ka��CH3COOH��=1.7��10-5 mol/L��H2CO3�ĵ��볣��Ka1=4.2��10-7mol•L-1��Ka2=5.6��10-11mol•L-1��1�����������ӷ���ʽ����̼������ˮ��Һ�Լ��Ե�ԭ��HCO3-+H2O?H2CO3+OH-��
�ڳ����£����ʵ���Ũ����ͬ������������Һ��
a��̼������Һ b����������Һ c������������Һ d������������Һ��
��pH�ɴ�С��˳���ǣ�dcab������ţ���
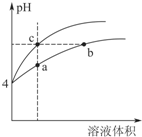
��2��ij�¶��£�pH��Ϊ4������ʹ�����Һ�ֱ��ˮϡ�ͣ���pH����Һ����仯��������ͼ4������a��b��c�����Ӧ����Һ��ˮ�ĵ���̶��ɴ�С��˳����b=c��a���ô�����Һϡ�����У�����
����һ����С����bd��
a��c��OH-�� b�� c��H+��
c.$\frac{c��C{H}_{3}COOH��c��O{H}^{-}��}{c��C{H}_{3}CO{O}^{-}��}$ d��$\frac{c��C{H}_{3}COOH��}{c��{H}^{+}��}$
��3����0.10mol•L-1NaOHΪ��Һ���ⶨij�����Ũ�ȣ�ȡ20.00mL����������Һ������ƿ�У����μ�2��3�η�̪��ָʾ������NaOH����Һ���еζ����ظ������ζ�����2��3�Σ���¼�������£�
| ʵ���� | ����������Һ��Ũ�� ��mol•L-1�� | �ζ����ʱ������������Һ����������mL�� | ��������������mL�� |
| 1 | 0.10 | 24.12 | 20.00 |
| 2 | 0.10 | 23.88 | 20.00 |
| 3 | 0.10 | 24.00 | 20.00 |
�ڸ����������ݣ��ɼ�����������Ũ��ԼΪ0.12mol•L-1 mol•L-1
��4����t��ʱ��ijNaOHϡ��Һ��c��H+��=10-a mol•L-1��c��OH-��=10-b mol•L-1����֪a+b=13����
�ٸ��¶���ˮ�����ӻ�����Kw=10-13mol2•L-2��
���ڸ��¶��£���100mL0.1mol•L-1��ϡH2SO4��100mL0.4mol•L-1��NaOH��Һ��Ϻ���Һ��pH=12��
| A�� | �еĹ��ۻ�������Һ̬ʱҲ�ܵ��� | |
| B�� | �������Ӽ��Ļ������У�Ҳ���ܻ����й��ۼ� | |
| C�� | �γ����Ӽ����������ǽ����������������� | |
| D�� | �ǽ���Ԫ���γɵĻ�����һ�����������Ӽ� |
 ij�о���ѧϰС��̽���������Һ���������������ʵ�飮
ij�о���ѧϰС��̽���������Һ���������������ʵ�飮��1��ȡһ�����ı���������250mL 0.5000mol•L-1������Һʱ��Ҫ�õ��IJ�����������Ͳ���ձ�����������250mL����ƿ�ͽ�ͷ�ιܣ�
��2��������0.5000mol•L-1�Ĵ�����Һ�ٽ���ϡ�ͣ�Ϊ�ⶨϡ�ͺ������Һ��ȷŨ�ȣ���0.2000mol•L-1��NaOH��Һ��25.00mL������Һ���еζ������εζ�����NaOH��Һ��������£�
| ʵ����� | 1 | 2 | 3 | 4 |
| ����NaOH��Һ�������mL�� | 25.05 | 25.00 | 23.80 | 24.95 |
��3��ʵ�飨2���У��ζ�������pH�仯������ͼ��ʾ�������£���
�ٵζ������У����μ�12.50mLNaOHʱ�����û����Һ������Ũ���ɴ�С˳��Ϊc��CH3COO-����c��Na+����c��H+����c��OH-����
�ڵ��μ�25.00mLNaOHʱ����Ӧ���û����Һ��pH=9��������Һ�У�ˮ�ĵ�����Ǵ�ˮ��100����
c��OH-��-c��CH3COOH��=10-9mol•L-1��
����Ȼ�� �ں��� ��ʯ�� ��̫���� �ݷ��� �����ܣ�
| A�� | �٢ڢ� | B�� | �ܢݢ� | C�� | �٢ۢ� | D�� | �ڢܢ� |
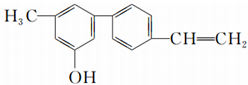
| A�� | ���л�������ϩ�� | B�� | ���л������ڴ� | ||
| C�� | ���л�����������е�ԭ�Ӷ����� | D�� | ���л��������ֹ����� |