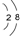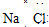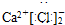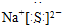��Ŀ����
ij��ˮ�������������е�5�֣�����ˮ�ĵ��뼰���ӵ�ˮ�⣩��K+��Cu2+��Al3+��Fe2+��Cl-��CO32-��NO3-��SO42-����ø������ӵ����ʵ���Ũ����ȣ�Ϊ̽����ˮ����ɣ�ijͬѧ����������ʵ�飺
���ò�˿պȡ������Һ���ڻ��������գ�����ɫ�ܲ����۲�����ɫ���森
����ȡ��Һ��������ϡ���ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����ʱ��Һ��Ȼ���壬����Һ������������䣮
����ȡ��Һ����BaCl2��Һ���а�ɫ�������ɣ�
��������ʵ�飬�����Ʋ���ȷ���ǣ�������
���ò�˿պȡ������Һ���ڻ��������գ�����ɫ�ܲ����۲�����ɫ���森
����ȡ��Һ��������ϡ���ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����ʱ��Һ��Ȼ���壬����Һ������������䣮
����ȡ��Һ����BaCl2��Һ���а�ɫ�������ɣ�
��������ʵ�飬�����Ʋ���ȷ���ǣ�������
| A����Һ����ȷ��Al3+�Ĵ������ |
| B��ԭ��Һ�в���������Ϊ��K+��Al3+��CO32- |
| C��������п���ȷ��Fe2+��NO3-�Ĵ��� |
| D��������й���2�ֱ��γ��� |
���㣺���������ӵļ���,���������ӵļ���
ר�⣺���ʼ��������
�������ò�˿պȡ������Һ���ڻ��������գ�����ɫ�ܲ����۲�����ɫ���棬��˵��û��K+��
��ȡ��Һ��������ϡ���ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����˵�����о��������Ե�NO3-�ͻ�ԭ�Ե�Fe2+��
������Һ�м���BaCl2��Һ���а�ɫ�������ɣ�˵��ԭ��Һ�к���CO32-��SO42-����Ϊ��Fe2+��Fe2+��CO32-���ܹ��棬����û��CO32-��
����Һ�е�������Fe2+��NO3-��SO42-��
��֪�������ӵ����ʵ���Ũ����ȣ������ӵ�ɱ������ӵ�ɶ࣬���Ի��������������Cu2+��Al3+������Cu2+�������ӵĵ�ɶ����Cl-������Al3+�������Ӽ���Cl-������Բ��غ㣻
���Ը��ݵ���غ��֪����Cu2+��Cl-��
����ԭ��Һ������������Ϊ��Cu2+��Fe2+��Cl-��NO3-��SO42-��һ��������K+��Al3+��CO32-���ӣ��ݴ˷���ѡ�
��ȡ��Һ��������ϡ���ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����˵�����о��������Ե�NO3-�ͻ�ԭ�Ե�Fe2+��
������Һ�м���BaCl2��Һ���а�ɫ�������ɣ�˵��ԭ��Һ�к���CO32-��SO42-����Ϊ��Fe2+��Fe2+��CO32-���ܹ��棬����û��CO32-��
����Һ�е�������Fe2+��NO3-��SO42-��
��֪�������ӵ����ʵ���Ũ����ȣ������ӵ�ɱ������ӵ�ɶ࣬���Ի��������������Cu2+��Al3+������Cu2+�������ӵĵ�ɶ����Cl-������Al3+�������Ӽ���Cl-������Բ��غ㣻
���Ը��ݵ���غ��֪����Cu2+��Cl-��
����ԭ��Һ������������Ϊ��Cu2+��Fe2+��Cl-��NO3-��SO42-��һ��������K+��Al3+��CO32-���ӣ��ݴ˷���ѡ�
���
�⣺�ò�˿պȡ������Һ���ڻ��������գ�����ɫ�ܲ����۲�����ɫ���棬��˵��û��K+��
��ȡ��Һ��������ϡ���ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����˵�����о��������Ե�NO3-�ͻ�ԭ�Ե�Fe2+��
������Һ�м���BaCl2��Һ���а�ɫ�������ɣ�˵��ԭ��Һ�к���CO32-��SO42-����Ϊ��Fe2+��Fe2+��CO32-���ܹ��棬����û��CO32-��
����Һ�е�������Fe2+��NO3-��SO42-��
��֪�������ӵ����ʵ���Ũ����ȣ������ӵ�ɱ������ӵ�ɶ࣬���Ի��������������Cu2+��Al3+������Cu2+�������ӵĵ�ɶ����Cl-������Al3+�������Ӽ���Cl-������Բ��غ㣻
���Ը��ݵ���غ��֪����Cu2+��Cl-��
����ԭ��Һ������������Ϊ��Cu2+��Fe2+��Cl-��NO3-��SO42-��һ��������K+��Al3+��CO32-���ӣ�
A���������Ϸ�����ȷ��һ����Al3+�Ĵ��ڣ���A����
B���������Ϸ�����ԭ��Һ�в���������Ϊ��K+��Al3+��CO32-����B��ȷ��
C����ȡ��Һ��������ϡ���ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����˵�����о��������Ե�NO3-�ͻ�ԭ�Ե�Fe2+����C��ȷ��
D����ȡ��Һ����BaCl2��Һ���������ᱵ��������D����
��ѡBC��
��ȡ��Һ��������ϡ���ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����˵�����о��������Ե�NO3-�ͻ�ԭ�Ե�Fe2+��
������Һ�м���BaCl2��Һ���а�ɫ�������ɣ�˵��ԭ��Һ�к���CO32-��SO42-����Ϊ��Fe2+��Fe2+��CO32-���ܹ��棬����û��CO32-��
����Һ�е�������Fe2+��NO3-��SO42-��
��֪�������ӵ����ʵ���Ũ����ȣ������ӵ�ɱ������ӵ�ɶ࣬���Ի��������������Cu2+��Al3+������Cu2+�������ӵĵ�ɶ����Cl-������Al3+�������Ӽ���Cl-������Բ��غ㣻
���Ը��ݵ���غ��֪����Cu2+��Cl-��
����ԭ��Һ������������Ϊ��Cu2+��Fe2+��Cl-��NO3-��SO42-��һ��������K+��Al3+��CO32-���ӣ�
A���������Ϸ�����ȷ��һ����Al3+�Ĵ��ڣ���A����
B���������Ϸ�����ԭ��Һ�в���������Ϊ��K+��Al3+��CO32-����B��ȷ��
C����ȡ��Һ��������ϡ���ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����˵�����о��������Ե�NO3-�ͻ�ԭ�Ե�Fe2+����C��ȷ��
D����ȡ��Һ����BaCl2��Һ���������ᱵ��������D����
��ѡBC��
���������⿼�������Ӽ����ʵ�鷽���ͷ�Ӧ��������������������ʺ���Һ�е���غ�����жϴ��ڵ������ǽ���ؼ�����Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
�����Ŀ
����ʵ������������ȷ���ǣ�������
A�� �ռ����� |
B�� ģ��ʯ������ |
C�� ת����Һ |
D�� �ú�ˮ����������ˮ |
һ���¶Ⱥ�ѹǿ�£�N2��g����H2��g����Ӧ����2molNH3��g�����ų�92.4kJ����������ͬ�¶��£���һ��ѹ������ͨ�� 1molN2��3molH2����ƽ��ʱ�ų�����ΪQ1kJ������һ�����ͬ����������ͨ��1molN2��3molH2����ͬ�¶��´ﵽƽ��ʱ�ų�����ΪQ2kJ��������������ȷ���ǣ�������
| A��Q2��Q1=92.4kJ |
| B��Q2=Q1=92.4kJ |
| C��Q2��Q1��92.4kJ |
| D��Q2=Q1��92.4kJ |
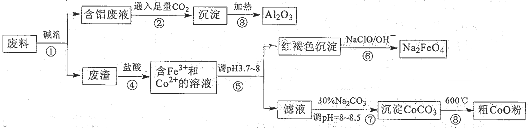
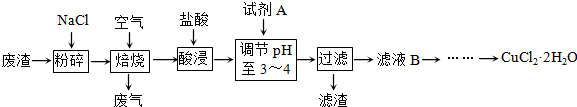
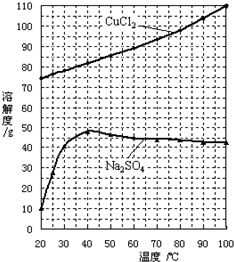
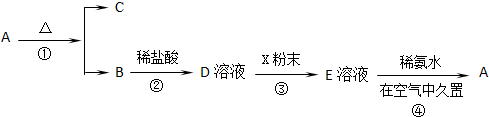
 ��������Ԫ�أ�����A��B��C��D��EΪ������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش��������⣮
��������Ԫ�أ�����A��B��C��D��EΪ������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش��������⣮ ��ͬѧ�����ĵ����Ų�ͼΥ����
��ͬѧ�����ĵ����Ų�ͼΥ����