��Ŀ����
4��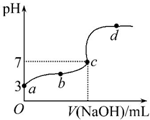 ��֪��25����������10.00mL 0.1mol•L-1HCOOH��Һ����μ���0.1mol•L-1NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯��������˵���в���ȷ���ǣ�������
��֪��25����������10.00mL 0.1mol•L-1HCOOH��Һ����μ���0.1mol•L-1NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯��������˵���в���ȷ���ǣ�������| A�� | a���ʾ����Һ��c��HCOO-��ԼΪ10-3mol•L-1 | |
| B�� | ��25 mL����ʽ�ζ�����ȡHCOOH��Һ | |
| C�� | c��NaOH��Һ�����С��10 mL | |
| D�� | ��a��c����һ�㣬��Һ��һ������c��HCOO-����c��Na+����c��H+����c��OH-�� |
���� A��a��ʱ��ҺpH=3��������Ũ��Ϊ10-3 mol•L-1����Һ�м��������Ũ��ԼΪ10-3 mol•L-1��
B������Ϊ������Һ����Ҫʹ����ʽ�ζ�����ȡ��
C��������������ҺΪ10mL������ǡ�÷�Ӧ���ɼ����ƣ���Һ��ʾ���ԣ���c����ҺpH=7������Ӧ�Թ���Щ��
D�����ݵ���غ�����жϣ���a��c����һ�㣺c��H+����c��OH-�������ݵ���غ��֪��c��Na+����c��HCOO-����
��� �⣺A�����ڼ�����ˮ�����������Ũ�Ⱥ�С��������Һ��������Ũ����������ļ��������Ũ�Ȼ�����ȣ�a��ʱ��ҺpH=3��������Ũ��Ϊ10-3 mol•L-1���������Һ�м��������Ũ��ԼΪ10-3 mol•L-1����A��ȷ��
B������ΪһԪ���ᣬ����ȡ�ü���Ӧ��ʹ����ʽ�ζ��ܣ���B��ȷ��
C�����ڼ���Ϊ���ᣬ������10mL����������Һʱ����Ӧ����ǿ�������μ����ƣ���Ӧ�����ҺΪ���ԣ�����c����ʾ���ԣ�����������������Һ���Ӧ��С��10mL����C��ȷ��
D����a��c����һ�㣬��Һ��ʾ���ԣ���һ�����㣺c��H+����c��OH-�������ݵ���غ㣺c��Na+��+c��H+��=c��HCOO-��+c��OH-����֪��c��Na+����c��HCOO-������������Ũ����ȷ��ϵΪ��c��HCOO-����c��Na+����c��H+����c��OH-������D����
��ѡD��
���� ���⿼��������Ϻ���Һ������жϼ���ҺpH�ļ��㣬��Ŀ�Ѷ��еȣ�ע��������Һ���������ҺpH�Ĺ�ϵ���ܹ����ݵ���غ㡢�ε�ˮ���ж���Һ������Ũ�ȴ�С��

| A�� | ҽ�þƾ���ָ��������Ϊ75%���Ҵ���Һ | |
| B�� | �ù��˵ķ������Է����������������� | |
| C�� | �����ǡ���������һ�������¶��ܷ���������Ӧ | |
| D�� | �ڵ�������Һ�м���Ũ�����Σ��磨NH4��2SO4��CuSO4�ȣ�����ʹ�����ʵ��ܽ�Ƚ��Ͷ�������������̳�֮Ϊ���� |
| A�� | ��aL0.1mol/L��CH3COOH��Һ��bL0.1mol/L��KOH��Һ��ϣ�������Һ��һ�����ڣ�c��K+��+c��H+���Tc��CH3COO-��+c��OH-�� | |
| B�� | ��0.1mol/L��NaHCO3��Һ��0.3mol/L��Ba��OH��2��Һ�������ϣ�������Һ��һ�����ڣ�c��OH-����c��Ba+����c��Na+����c��H+�� | |
| C�� | ��1mol/L��CH3COOH��Һ�м�������CH3COONa���壬����CH3COONaˮ���Լ��ԣ�������Һ��pH���� | |
| D�� | �����£���pH=3��CH3COOH��Һ��pH=11��NaOH��Һ�У�ˮ�ĵ���̶���ͬ�� |
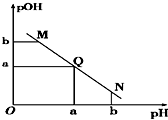 ij�¶��£���һ�����1mol/L������Һ����μ����Ũ�ȵ�NaOH��Һ����Һ��pOH��pOH=-lg c[OH-]����pH�ı仯��ϵ��ͼ��ʾ��������
ij�¶��£���һ�����1mol/L������Һ����μ����Ũ�ȵ�NaOH��Һ����Һ��pOH��pOH=-lg c[OH-]����pH�ı仯��ϵ��ͼ��ʾ��������| A�� | M����ʾ��Һ��������ǿ��Q�� | |
| B�� | N����ʾ��Һ��c��CH3COO-����c��Na+�� | |
| C�� | M���N����ʾ��Һ��ˮ�ĵ���̶Ȳ���ͬ | |
| D�� | Q������NaOH��Һ�����С�ڴ�����Һ����� |
| A�� | ���ɵ����λ�ѧʽΪBmAn | |
| B�� | �����д�����һ��ˮ������ӣ�������ˮ�ⷽ��ʽΪ��Bm++mH2O?B��OH��m+mH+ | |
| C�� | ���ɵ���Ϊǿ�������� | |
| D�� | HnAΪ���ᣬ���һ�����뷽��ʽΪ��HmA?Hm-1A-+H+ |
| A�� | CH3Br | B�� | CH3CH2CH2CH2Br | ||
| C�� | CH3CHBrCH2Br | D�� | BrCH2CH2CH2CH2Br |
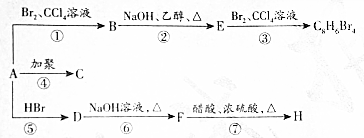
 ��D
��D ��E
��E ��
�� +2NaOH$��_{��}^{��}$
+2NaOH$��_{��}^{��}$ +NaBr��ȡ����Ӧ����
+NaBr��ȡ����Ӧ���� ABCDE������Һ�ֱ���NaOH��Һ����ˮ�����ᡢ���ᡢNH4HSO4��Һ��һ�֣������½�������ʵ�飺
ABCDE������Һ�ֱ���NaOH��Һ����ˮ�����ᡢ���ᡢNH4HSO4��Һ��һ�֣������½�������ʵ�飺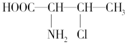 ��һ�����л�������к���2������̼ԭ�ӣ�����-NH2��N���ӻ����������sp3��
��һ�����л�������к���2������̼ԭ�ӣ�����-NH2��N���ӻ����������sp3��