��Ŀ����
 ͨ����ʳ���Ϳɻ��ij�����л���X������Է�������Ϊ46������̼����������Ϊ52.2%�������������Ϊ13.0%��
ͨ����ʳ���Ϳɻ��ij�����л���X������Է�������Ϊ46������̼����������Ϊ52.2%�������������Ϊ13.0%����1��X������е�������ͭ���·�Ӧ����Y����ѧ����ʽΪ
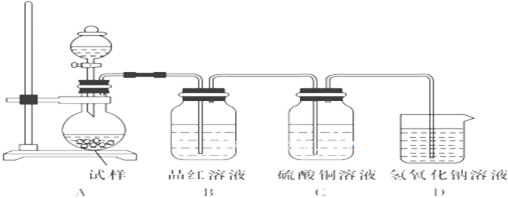
��2��X�����Ը��������Һ��Ӧ������Z���ڼ��Ⱥ�Ũ���������£�X��Z��Ӧ������һ������ζ������W��ijͬѧ������ͼ��ʾ��ʵ��װ����ȡW��ʵ��������Թܼ����ϲ�Ϊ��ɫ���ġ�������ˮ����״Һ�壮
��ʵ�鿪ʼʱ���Թܼ��еĵ��ܲ�����Һ���µ�ԭ���ǣ�
����������Թܼ��и���״Һ����Ҫ�õ���������
a��©�� b����Һ©�� c������©��
��ʵ������������Թܼף�������ɫ�������ɣ�����Ҫԭ���ǣ�
���㣺������������ȡ,�Ҵ��Ļ�ѧ����
ר�⣺
��������1��ͨ����ʳ���Ϳɻ��ij�����л���X������Է�������Ϊ46������̼����������Ϊ52.2%�������������Ϊ13.0%����������������Ϊ1-52.2%-13.0%=34.8%���ݴ˵ó�����ʽΪC2H6O��Ϊ�Ҵ���
��2��X�����Ը��������Һ��Ӧ������Z���ڼ��Ⱥ�Ũ���������£�X��Z��Ӧ������һ������ζ������W����ZΪCH3COOH��WΪCH3COOCH2CH3��Ϊ�����������Ʊ���
��2��X�����Ը��������Һ��Ӧ������Z���ڼ��Ⱥ�Ũ���������£�X��Z��Ӧ������һ������ζ������W����ZΪCH3COOH��WΪCH3COOCH2CH3��Ϊ�����������Ʊ���
���
�⣺��1��ͨ����ʳ���Ϳɻ��ij�����л���X������Է�������Ϊ46������̼����������Ϊ52.2%�������������Ϊ13.0%����������������Ϊ1-52.2%-13��%=34.8%�����л����к�C��
=2����H��
=6����O��
=1�������ʽΪC2H6O��Ϊ�Ҵ���CH3CH2OH������е�������ͭ���·�Ӧ����YΪCH3CHO��
��ѧ����ʽΪ2CH3CH2OH+O2
2CH3CHO+2H2O��
�ʴ�Ϊ��2CH3CH2OH+O2
2CH3CHO+2H2O��
��2��X�����Ը��������Һ��Ӧ������Z���ڼ��Ⱥ�Ũ���������£�X��Z��Ӧ������һ������ζ������W����ZΪCH3COOH��WΪCH3COOCH2CH3��Ϊ�����������Ʊ���
�������ﺬ���Ҵ������ᣬ���߶�������ˮ�������ò��뵽Һ�����£���������������ʴ�Ϊ����ֹ��Һ������
�����ɵ��������������ڱ���̼������Һ��Ӧ�÷�Һ�ķ������룬�ʴ�Ϊ��b��
�������ӷ����������ԣ�����̼���Ʒ���2CH3COOH+Na2CO3=2CH3COONa+H2O+CO2�����ʴ�Ϊ��2CH3COOH+Na2CO3=2CH3COONa+H2O+CO2����
| 46��52.2% |
| 12 |
| 46��13.0% |
| 1 |
| 46��34.8% |
| 16 |
��ѧ����ʽΪ2CH3CH2OH+O2
| Cu |
| �� |
�ʴ�Ϊ��2CH3CH2OH+O2
| Cu |
| �� |
��2��X�����Ը��������Һ��Ӧ������Z���ڼ��Ⱥ�Ũ���������£�X��Z��Ӧ������һ������ζ������W����ZΪCH3COOH��WΪCH3COOCH2CH3��Ϊ�����������Ʊ���
�������ﺬ���Ҵ������ᣬ���߶�������ˮ�������ò��뵽Һ�����£���������������ʴ�Ϊ����ֹ��Һ������
�����ɵ��������������ڱ���̼������Һ��Ӧ�÷�Һ�ķ������룬�ʴ�Ϊ��b��
�������ӷ����������ԣ�����̼���Ʒ���2CH3COOH+Na2CO3=2CH3COONa+H2O+CO2�����ʴ�Ϊ��2CH3COOH+Na2CO3=2CH3COONa+H2O+CO2����
���������⿼���л�����ƶϣ�������ѧ����������������������ʵ�������Ŀ��飬ע����ճ����л���Ľṹ�����ʣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
 ���ֶ�����Ԫ��A��B��C��D��Ԫ�����ڱ��е����λ����ͼ��ʾ������D�γɵ����������ﶼ�Ǵ�����Ⱦ������й��ж���ȷ���ǣ�������
���ֶ�����Ԫ��A��B��C��D��Ԫ�����ڱ��е����λ����ͼ��ʾ������D�γɵ����������ﶼ�Ǵ�����Ⱦ������й��ж���ȷ���ǣ�������| A�����⻯������ȶ��ԣ�C��A |
| B������������Ӧˮ��������ԣ�D��C |
| C��B������������ˮ��������D������������ˮ���ﷴӦ |
| D��A��C��D����ۺ����������ˮ��Һ���ܾ��Լ��� |
ijǿ������Һ�п��ܴ���NO3-��I-��Cl-��Fe3+�е�һ�ֻ��֣������Һ�м�����ˮ���屻��ԭ���ɴ��ƶϸ���Һ�У�������
| A������NO3-��Ҳ����Fe3+ |
| B������NO3-��I-��Cl? |
| C����I-���ҿ϶�����Cl- |
| D�����ܺ���Fe3+ |
������ʵ���У�������������ȷ���ǣ�������
A����������ƿ��ʢԼ
| ||
| B���ռ�����ˮʱ��Ӧ��ȥ��ʼ����IJ��� | ||
| C����ˮ���������¿��룬�Ͽڳ� | ||
| D�����¶ȼ�ˮ�����������ˮ�� |