��Ŀ����
������ѧij��ѧ��ȤС�������ͼ��ʾ��ʵ��װ����̽�������������Ⱥ��������Ʒֽ��IJ���������ϣ���ˮ�������Ƹ����������ȵ�600��ſ�ʼ�ֽ⣩��
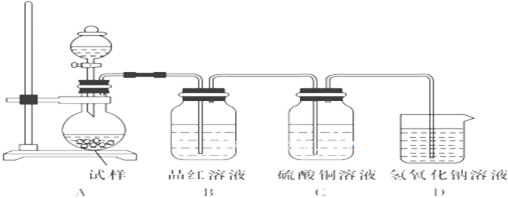
��1����μ������װ�õ������� ��
��2����������¶ȵ���600�棬��������ȴ����������еμ�70%��������������װ��B�й۲쵽������Ϊ ����ʱA�з�Ӧ�����ӷ���ʽΪ�� ��
��3���������¶�Ϊ600������һ�������������ȴ����������л����μ�ϡ�������������۲쵽��ƿ�г��ֵ���ɫ������ͬʱ������B������������C�з����к�ɫ���������������ɵ���ɫ���������ӷ���ʽΪ ��
��4���ڣ�3���еμ�����ϡ�������ƿ�ڳ�Cl-�⣬��������һ��Ũ�Ƚϴ�������ӣ��������������ӵķ����� ��
��5��д��Na2SO3������ȵ�600�����Ϸֽ�Ļ�ѧ����ʽ�� ��

��1����μ������װ�õ�������
��2����������¶ȵ���600�棬��������ȴ����������еμ�70%��������������װ��B�й۲쵽������Ϊ
��3���������¶�Ϊ600������һ�������������ȴ����������л����μ�ϡ�������������۲쵽��ƿ�г��ֵ���ɫ������ͬʱ������B������������C�з����к�ɫ���������������ɵ���ɫ���������ӷ���ʽΪ
��4���ڣ�3���еμ�����ϡ�������ƿ�ڳ�Cl-�⣬��������һ��Ũ�Ƚϴ�������ӣ��������������ӵķ�����
��5��д��Na2SO3������ȵ�600�����Ϸֽ�Ļ�ѧ����ʽ��
���㣺ʵ��װ���ۺ�
ר�⣺ʵ�������
��������1������װ���ܱպ�����ð����Һ��仯�����ж�װ�������ԣ�
��2�������¶ȵ���600�棬�������Ʋ��ֽ⣬����������ɶ�������
��3��SO32-��S2-��������������ת��Ϊ������Ʒ����Һ����ɫ������SO32-+2S2-+6H+=3S��+3H2O��
��4���������Ʒֽ�����к������ƣ�������Ԫ�صĻ��ϼ۱仯��֪����һ�ֲ�������Ԫ�صĻ��ϼ����ߣ�Ϊ�����ƣ�
��5���ɣ�4��������֪Na2SO3������ȵ�600�����Ϸֽ������Na2SO4��Na2S��
��2�������¶ȵ���600�棬�������Ʋ��ֽ⣬����������ɶ�������
��3��SO32-��S2-��������������ת��Ϊ������Ʒ����Һ����ɫ������SO32-+2S2-+6H+=3S��+3H2O��
��4���������Ʒֽ�����к������ƣ�������Ԫ�صĻ��ϼ۱仯��֪����һ�ֲ�������Ԫ�صĻ��ϼ����ߣ�Ϊ�����ƣ�
��5���ɣ�4��������֪Na2SO3������ȵ�600�����Ϸֽ������Na2SO4��Na2S��
���
�⣺��1���رշ�Һ©���Ļ�������װ��D�м�һ������ˮ��ʹ����װ���γ��ܱ�ϵͳ������ë������װ��A�е���ƿ��ƿ�������������ͣ����������ᷢ��D�е���������ð������ȴ�����������С������ѹѹ��ˮ���뵼�ܣ���D�е������γ�һ��ˮ����˵����װ�ò�©������װ�õ����������ã�
�ʴ�Ϊ���رշ�Һ©���Ļ�������װ��D�м�һ������ˮ������ë������װ��A�е���ƿ������D�е���������ð������ȴ���ַ���D�е����γ�һ��ˮ����˵����װ�õ����������ã�
��2�������¶ȵ���600�棬�������Ʋ��ֽ⣬����������ɶ����������������Ư���ԣ���ʹƷ����Һ��ɫ���������������ᷴӦ�����ӷ���ʽΪ��SO32-+2H+=SO2��+H2O��
�ʴ�Ϊ��Ʒ����Һ��ɫ��SO32-+2H+=SO2��+H2O��
��3���¶ȸ���600��ʱ��Na2SO3��ʼ�ֽ⣬���ù��������Na2SO3��Na2S�ȵĻ�������ϡ����ʱ��SO32-��S2-��������������ת��Ϊ�������ݡ�����ͭ�г��ֺ�ɫ�������ɵó�����ƿ��û��SO2�ų���������Һ���ֵ������ǣ��е���ɫ�������ɲ�������ð��������ΪS������Ϊ���⣬��������Һ�з�����Ӧ�����ӷ���ʽΪSO32-+2S2-+6H+=3S��+3H2O��S2-+2H+=H2S����
�ʴ�Ϊ��SO32-+2S2-+6H+=3S��+3H2O��S2-+2H+=H2S����
��4���������Ʒֽ�����к������ƣ���Ԫ�صĻ��ϼ۽��ͣ�����һ�ֲ�������Ԫ�صĻ��ϼ����ߣ�Ϊ�����ƣ�Ҫ������������ӵĴ��ڣ���ȡ������������ˮ�����Һ��ȡ������Һ���Թ��У��ȼ�ϡ���ᣬ�������������ټ��Ȼ�����Һ�����а�ɫ�������ɣ�˵��SO42-�д��ڣ�
�ʴ�Ϊ����ȡ������������ˮ�����Һ��ȡ������Һ���Թ��У��ȼ�ϡ���ᣬ�������������ټ��Ȼ�����Һ�����а�ɫ�������ɣ�˵��SO42-�д��ڣ�
��5���ɣ�4��������֪Na2SO3������ȵ�600�����Ϸֽ������Na2SO4��Na2S����Ӧ�ķ���ʽΪ��4Na2SO3
Na2S+3Na2SO4��
�ʴ�Ϊ��4Na2SO3
Na2S+3Na2SO4��
�ʴ�Ϊ���رշ�Һ©���Ļ�������װ��D�м�һ������ˮ������ë������װ��A�е���ƿ������D�е���������ð������ȴ���ַ���D�е����γ�һ��ˮ����˵����װ�õ����������ã�
��2�������¶ȵ���600�棬�������Ʋ��ֽ⣬����������ɶ����������������Ư���ԣ���ʹƷ����Һ��ɫ���������������ᷴӦ�����ӷ���ʽΪ��SO32-+2H+=SO2��+H2O��
�ʴ�Ϊ��Ʒ����Һ��ɫ��SO32-+2H+=SO2��+H2O��
��3���¶ȸ���600��ʱ��Na2SO3��ʼ�ֽ⣬���ù��������Na2SO3��Na2S�ȵĻ�������ϡ����ʱ��SO32-��S2-��������������ת��Ϊ�������ݡ�����ͭ�г��ֺ�ɫ�������ɵó�����ƿ��û��SO2�ų���������Һ���ֵ������ǣ��е���ɫ�������ɲ�������ð��������ΪS������Ϊ���⣬��������Һ�з�����Ӧ�����ӷ���ʽΪSO32-+2S2-+6H+=3S��+3H2O��S2-+2H+=H2S����
�ʴ�Ϊ��SO32-+2S2-+6H+=3S��+3H2O��S2-+2H+=H2S����
��4���������Ʒֽ�����к������ƣ���Ԫ�صĻ��ϼ۽��ͣ�����һ�ֲ�������Ԫ�صĻ��ϼ����ߣ�Ϊ�����ƣ�Ҫ������������ӵĴ��ڣ���ȡ������������ˮ�����Һ��ȡ������Һ���Թ��У��ȼ�ϡ���ᣬ�������������ټ��Ȼ�����Һ�����а�ɫ�������ɣ�˵��SO42-�д��ڣ�
�ʴ�Ϊ����ȡ������������ˮ�����Һ��ȡ������Һ���Թ��У��ȼ�ϡ���ᣬ�������������ټ��Ȼ�����Һ�����а�ɫ�������ɣ�˵��SO42-�д��ڣ�
��5���ɣ�4��������֪Na2SO3������ȵ�600�����Ϸֽ������Na2SO4��Na2S����Ӧ�ķ���ʽΪ��4Na2SO3
| ||
�ʴ�Ϊ��4Na2SO3
| ||
���������⿼������ʵ�鷽������ƣ�ע��ʵ������ķ����Ʋ⡢ʵ�������о����������⣬���ط�����ʵ���������ۺϿ��飬��Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
���й�����������˵����ȷ���ǣ�������
| A��0.012kg C-12��12C�������е�̼ԭ�����ʵ���Ϊ1mol |
| B��SO42-��Ħ�������� 98 g?mol-1 |
| C��1 mol�κ�������ռ�����Լ��22.4 L |
| D�������ӵ���������6.02��1023 mol-1 |
����˵����ȷ���ǣ�������
| A����100�桢101 KPa�����£�1molҺ̬ˮ����Ϊˮ�������յ�����Ϊ40.69KJ����H2O��g��?H2O��l�� �ġ�H=-40.69KJ/mol |
| B����֪MgCO3��Ksp=6.82��10-4mol2/L2�������к�����MgCO3����Һ�У�����C��Mg 2+ ��=C��CO32-������ C��Mg2+��?C��CO32-��=6.82��10-4mol2/L2 |
| C����֪��C-C�ļ���348KJ/mol��C=C�ļ���610KJ/mol��C-H�ļ���413KJ/mol�� H-H�ļ���436KJ/mol��  ���ʱ�Ϊ����H=[��4��348+3��610+8��413��+3��436-��7��348+14��413��]=-384 kJ/mol ���ʱ�Ϊ����H=[��4��348+3��610+8��413��+3��436-��7��348+14��413��]=-384 kJ/mol |
| D��̼��������Һ�д��ڣ�c��H*��+c��H2CO3��=c��OH-��+c��CO32-�� |
 ��SO2ͨ��BaCl2��Һ����������������ͨ����һ���������Բ�����ɫ���������Ҳ�Y�ι��з��õ�ҩƷ��ϲ�����Ҫ����ǣ���Ҫʱ���Լ��ȣ���������
��SO2ͨ��BaCl2��Һ����������������ͨ����һ���������Բ�����ɫ���������Ҳ�Y�ι��з��õ�ҩƷ��ϲ�����Ҫ����ǣ���Ҫʱ���Լ��ȣ���������| A��ʯ��ʯ��ϡ���� |
| B��CaO���Ȼ�� |
| C��Cu��Ũ���� |
| D��Na2O2��ʳ��ˮ |
 ���������У��Ӵ����ڵķ�ӦΪ��
���������У��Ӵ����ڵķ�ӦΪ��



 ͨ����ʳ���Ϳɻ��ij�����л���X������Է�������Ϊ46������̼����������Ϊ52.2%�������������Ϊ13.0%��
ͨ����ʳ���Ϳɻ��ij�����л���X������Է�������Ϊ46������̼����������Ϊ52.2%�������������Ϊ13.0%��