��Ŀ����
13����һ�������£�CH3COOH��Һ�д��ڵ���ƽ�⣺CH3COOH?CH3COO-+H+��H��0��1�������£���PH=5��ϡ������Һ�У�c��CH3COO?��=10-5-10-9mol/L����ʽ����Ҫ��������˵���У�����ʹ0.10mol/L CH3COOH�ĵ���̶�������ǣ�����ĸ��BCF
A����������0.10mol/L��ϡ����
B������CH3COOH��Һ
C����ˮϡ����0.010mol/L
D����������������
E�����������Ȼ��ƹ���
F����������0.10mol/L��NaOH��Һ
��2��PH��ͬ�Ģ�HCl��aq����H2SO4��aq����CH3COOH��aq����100ml�ֱ���0.10mol/L��NaOH��aq���кͣ�����NaOH��aq��������ֱ�ΪV1��V2��V3�������ɴ�С��˳����V3��V1=V2��
���� ��1�����ݴ���ĵ���ƽ��CH3COOH?H++CH3COO-�����㣬���ݵ���ƽ���ƶ���Ӱ��������������
����ĵ��������ȷ�Ӧ����ˮϡ�͡��������ȶ��ܴٽ�����ĵ��룻
��2��H2SO4��HClΪǿ�ᣬpH��ͬ�������ͬ��H2SO4��HCl��Һ�����������ʵ�����ͬ����Ҫ�������������ʵ�����ͬ��CH3COOHΪ������ʣ����ڵ���ƽ�⣬��ͬpHʱ��������ҺŨ�ȴ���������Ũ�ȣ�
��� �⣺��1��������Һ���������ɴ����ˮ���룬pH=5������Һ�У�ˮ�����c��OH-��=$\frac{Kw}{c��{H}^{+}��}$=10-9mol•L-1����ˮ�����c��H+��=10-9mol•L-1��������Һ�д�������c��H+��=10-5-10-9mol•L-1������c��CH3COO-��=c��H+��=10-5-10-9mol•L-1��
����ĵ��������ȷ�Ӧ����ˮϡ�͡��������ȶ��ܴٽ�����ĵ��룻
A����������0.10mol•L-1��ϡ���ᣬ��Һ��������Ũ���������ƴ���ĵ��룬�����ĵ���̶Ƚ��ͣ��ʴ���
B������ĵ��������ȷ�Ӧ������CH3COOH��Һ���ٽ�����ĵ��룬�����ĵ���̶�������ȷ��
C����ˮϡ����0.010mol•L-1���ٽ�����ĵ��룬�����ĵ���̶�������ȷ��
D���������������ᣬ����ĵ���ƽ��������Ӧ�����ƶ���������ĵ���̶Ƚ��ͣ��ʴ���
E�����������Ȼ��ƹ��壬��Ӱ��ƽ����ƶ����ı����ĵ��룬�ʴ���
F����������0.10mol•L-1��NaOH��Һ�����������Ӻ������ӷ�Ӧ����ˮ��������Ũ�Ƚ��ͣ��ٽ�����ĵ��룬�����ĵ���̶�������ȷ��
�ʴ�Ϊ��10-5-10-9��BCF��
��2�����������pH��H2SO4��HCl�У���ҺpH��ͬ����c��H+����ͬ���μӵ�Ũ�ȵ��������ƽ�����ǡ���кͣ���������������Һ�������ȣ�����V1=V2����CH3COOHΪ���ᣬ��pHʱ�������Ũ��ԶԶ����HCl��H2SO4���μӵ�Ũ�ȵ��������ƽ�����ǡ���кͣ��к������������������������������ӣ��������������������Һ�������������NaOH��Һ�������ϵΪ��V3��V1=V2��
�ʴ�Ϊ��V3��V1=V2��
���� ���⿼����������ʵĵ���ƽ���Լ�����ϵļ��㣬����������ˮ���������ʵ���Ŀ��飬��Ŀ�Ѷ��еȣ�ע���������ϵļ��㷽����

 ��ͼ����Уʵ���һ�ѧ�Լ�Ũ�����ǩ�ϵIJ������ݣ�����Ҫ480mL 1mol•L-1��ϡ���ᣮ�ø�Ũ���������ˮ���ƣ��ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ��ڲ����������ձ�������Ͳ��
��ͼ����Уʵ���һ�ѧ�Լ�Ũ�����ǩ�ϵIJ������ݣ�����Ҫ480mL 1mol•L-1��ϡ���ᣮ�ø�Ũ���������ˮ���ƣ��ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ��ڲ����������ձ�������Ͳ��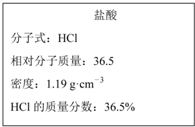 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺ ��������10mL 0.1mol•L-1 NaOH��Һ����μ���0.1mol•L-1��һԪ��HA����ҺpH�ı仯������ͼ��ʾ��
��������10mL 0.1mol•L-1 NaOH��Һ����μ���0.1mol•L-1��һԪ��HA����ҺpH�ı仯������ͼ��ʾ��