��Ŀ����
19����ҵ�Ͽ���N2 ��H2�ϳ�NH3����֪�÷�ӦΪ���ȷ�Ӧ����l��д���÷�Ӧ�Ļ�ѧ����ʽN2+3H2 $?_{����}^{���¡���ѹ}$2NH3��д�� NH3�ĵ���ʽ
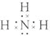
��2����֪1moIN2�еĹ��ۼ���������QlkJ��������1moIH2�еĹ��ۼ���������Q2kJ ���������γ�1moIN-H���ͷ�Q3kJ����������ϳɰ���Ӧ����1mol NH3ʱ�ų�������Ϊ$\frac{6{Q}_{3}-��{Q}_{1}+{3Q}_{2}��}{2}$kJ��
��3����ҵ�Ͽ�ͨ�����ķ�����N2��ȡNH3��2N2+6H2O$\frac{\underline{\;ͨ��\;}}{\;}$4NH3+3O2�����Ƶ�89.6L����״���£���NH3����ҪN22mol��ͬʱת��12mol���ӣ�
���� ��1�������������ڸ��¸�ѹ�����������·�����Ӧ�õ������������ǹ��ۻ����Nԭ�����������8���ӽṹ����ԭ��Ϊ2���ӽṹ��
��2�����ڷ��ȷ�Ӧ���ų�������Ϊ�ɼ��ų�����������ȥ�ϼ����յ���������
��3�����ݻ�ѧ����ʽ�ļ�����ϵ���㵪�����ʵ����Լ�ת�Ƶ��ӵ����ʵ�����
��� �⣺��1����ҵ�Ͽ�����N2��H2�ڸ��¸�ѹ�Լ������������ºϳ�NH3����N2+3H2 $?_{����}^{���¡���ѹ}$2NH3�������ǹ��ۻ���������ĵ���ʽΪ��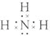 ���ʴ�Ϊ��N2+3H2 $?_{����}^{���¡���ѹ}$2NH3��
���ʴ�Ϊ��N2+3H2 $?_{����}^{���¡���ѹ}$2NH3��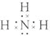 ��
��
��2��1molN2�Ĺ��ۼ��������յ�����ΪQ1kJ��1molH2�Ļ�ѧ���������յ�����ΪQ2kJ���γ�1moIN-H���ͷ�Q3kJ��1molNH3����3molN-H�����÷�Ӧ�Ƿ��ȷ�Ӧ�������»�ѧ���γ��ͷŵ���������ȥ�ɼ��������յ����������ڸ÷�Ӧ�ų������������ݷ���ʽ�ļ�����ϵ��֪������1mol NH3ʱ�ų�������Ϊ$\frac{6{Q}_{3}-��{Q}_{1}+{3Q}_{2}��}{2}$���ʴ�Ϊ��$\frac{6{Q}_{3}-��{Q}_{1}+{3Q}_{2}��}{2}$��
��3�����Ƶñ�״����89.6LNH3�����ʵ���Ϊ$\frac{89.6L}{22.4L/mol}$=4mol�����ݼ�����ϵ2N2��4NH3��12e-����֪��ҪN2���ʵ���Ϊ2mol��ת�Ƶ������ʵ���Ϊ12mol���ʴ�Ϊ��2��12��
���� ���⿼�黯ѧ����ʽ������ʽ����д����ѧ��Ӧ�е������仯����ѧ����ʽ���йؼ��㣬�����ڻ���֪ʶ�Ŀ��飬�ѶȲ���

 ������ȫ��������ϵ�д�
������ȫ��������ϵ�д�| A�� | ��״����2.24L O2 | |
| B�� | ��NA������ӵ�H2 | |
| C�� | 8.5g������������Ħ������Ϊ17g•mol-1�� | |
| D�� | ��3.01��1022�����ӵ�CH4 |
| t/min | ƽ��ʱ�������仯 | |||||||
| 0 | 20 | 40 | 60 | 80 | 120 | |||
| ��һ�� | X��HI�� | 0 | 0.60 | 0.73 | 0.773 | 0.780 | 0.784 | �ų�Q1kJ |
| �ڶ��� | X��HI�� | 1 | 0.91 | 0.85 | 0.815 | 0.795 | 0.784 | ����Q2kJ |
| A�� | Q1+Q2=11a��a��0�� | |
| B�� | k��=K•k����KΪ��֪��Ӧ��ƽ�ⳣ������ͬ�� | |
| C�� | K=$\frac{0.21{6}^{2}}{0.10{8}^{2}}$ | |
| D�� | ��ͬ�¶��£��ڵڶ���ƽ���Ļ���������ټ���2molHI�����´ﵽƽ���HI��ת����Ϊ21.6% |
��1��NO��һ����Ⱦ���ҵ����NH3��O2�ѳ�������NO��ԭ����ͼ��ʾ����ԭ����NO����ת��ΪN2���ѧʽ����V4+-O-H�ڷ�Ӧ��������������������2molNH3��0.5molO2ʱ����ȥ��NO�ڱ�״���µ����Ϊ44.8L��

��2��N2H4�Ƿ��亽�����ij���ȼ�ϣ���֪��N2H4��g��+O2��g��=N2��g��+2H2O��g����H=-538kJ•mol-1���Ͽ�1mol���л�ѧ��ϵ���������ֱ�ΪN-H��390kJ��N��N��946kJ��O-H��460kJ��O=O��498kJ����Ͽ�1molN-N��Ҫ���յ�������190kJ��
��3����ҵ������ʱ���ᷢ����Ӧ��4NH3��g��+5O2��g��$?_{����}^{����}$4NO��g��+6H2O��g����H��0��
���������������䣬������ͼ����ȷ����D������ĸ����

����1L�ݻ��̶����ܱ������г���NH3��O2�����÷�Ӧ���������ʵ�Ũ����ʱ��ı仯���±���ʾ��
 | c��NH3��/mol•L-1 | c��O2��/mol•L-1 | c��NO��mol•L-1 |
| 0 | 1.2 | 1.75 | 0 |
| 4min | 0.4 | 0.75 | 0.8 |
| 6min | 0.4 | 0.75 | 0.8 |
| 8min | 0.6 | 1 | 0.6 |
| 9min | 0.6 | 1 | 0.6 |
| 10min | 1.05 | 1 | 1.05 |
| 12min | �� | �� | �� |
| ��Ӧʱ��/min | n��CO��/mol | n��H2O��/mol |
| 0 | 1.20 | 0.60 |
| t1 | 0.80 | |
| t2 | 0.20 |
| A�� | ��Ӧ��t1 min�ڵ�ƽ������Ϊv��H2��=$\frac{0.40}{{t}_{1}}$mol/��L•min�� | |
| B�� | ���������������䣬��ʼʱ�������г���0.60 mol CO��1.20 mol H2������ƽ��ʱ��n��CO2��=0.40 mol | |
| C�� | ���������������䣬��ƽ����ϵ����ͨ��0.20 mol CO����ԭƽ����ȣ��ﵽ��ƽ��ʱCOת���ʼ�С��H2���������Ҳ��С | |
| D�� | �¶�����800�棬������Ӧƽ�ⳣ��Ϊ0.64��������ӦΪ���ȷ�Ӧ |
| A�� | ����Һ�еμ�Na2CO3��Һ�������������������� | |
| B�� | ��C��Cl-��=0.07mol/L������Һ��PHΪ2 | |
| C�� | ����Һ�м���NaOH��Һ�������ɵij����ʺ��ɫ | |
| D�� | ����Һ��ͨ��NH3��ֱ����Һ�����ԣ���ʱ��Һ��C��NH4+���TC��Cl-�� |
| A�� | �����£�AgCl��ͬ���ʵ���Ũ�ȵ�AgNO3��NaCl��Һ�е��ܽ�ȱȽϣ�ǰ�ߴ� | |
| B�� | �����£�pH=3�Ĵ�����Һ��pH=11��NaOH��Һ�������Ϻ��γ���Һa�������ʵ���Ũ�ȵ�������NaOH��Һ�������Ϻ��γ���Һb��ˮ�ĵ���̶�a��b | |
| C�� | ��������CO2ͨ��0.1 mol•L-1Na2CO3��Һ������Һǡ�ó����ԣ�����Һ�У���������Һ����仯��2c��CO32-��+c��HCO3-��=0.1 mol•L-1 | |
| D�� | �����£���֪�������ƽ�ⳣ��ΪKa�������ˮ��ƽ�ⳣ��ΪKh��ˮ�����ӻ�ΪKw�����У�Ka•Kh=Kw |
| A�� | BaSO4�������������ˮ����ȫ�����ܽ� | |
| B�� | �����ܽ�ﵽƽ��ʱ�����������ʺ��ܽ��������� | |
| C�� | �����ܽ�ﵽƽ��ʱ����Һ�����ʵ�����Ũ����ȣ��ұ��ֲ��� | |
| D�� | �����ܽ�ﵽƽ��ʱ������ټ��������Եĸó�������ٽ��ܽ� |
 ��֪ij���淴Ӧ���ܱ������н��У�A��g��+2B��g��?3C��g��+D��s����H��0��A��ת������ʱ��t��ϵ��ͼ��ʾ��ͼ������a����һ�������¸÷�Ӧ�Ĺ��̣���ʹa���߱�Ϊb���ߣ����ܲ�ȡ�Ĵ�ʩ�ǣ�������
��֪ij���淴Ӧ���ܱ������н��У�A��g��+2B��g��?3C��g��+D��s����H��0��A��ת������ʱ��t��ϵ��ͼ��ʾ��ͼ������a����һ�������¸÷�Ӧ�Ĺ��̣���ʹa���߱�Ϊb���ߣ����ܲ�ȡ�Ĵ�ʩ�ǣ�������| A�� | ����A��Ũ�Ȼ����C��Ũ�� | B�� | ��С�������ݻ��������� | ||
| C�� | �����¶Ȼ�����ѹǿ | D�� | ���߲���D |