��Ŀ����
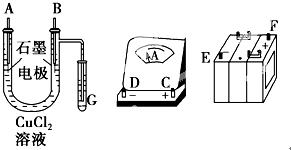 ijѧ����ͼ�õ�ⷨ���ݵ缫���������ʵ���������֤�����ӵ�����ֵ����ʵ�鷽����Ҫ��Ϊ��
ijѧ����ͼ�õ�ⷨ���ݵ缫���������ʵ���������֤�����ӵ�����ֵ����ʵ�鷽����Ҫ��Ϊ������ֱ�������Ȼ�ͭ��Һ������������ͼ��
���ڵ���ǿ��ΪI A��ͨ��ʱ��Ϊt s��ȷ�����缫��������ͭ������Ϊm g��
�Իش�
��1��������Щ��������ȷ˳��Ϊ����ͼ�б�ע������������Ӣ����ĸ��ʾ����ͬ����E��D��C��
��2��д��B���Ϸ�����Ӧ�ĵ缫��Ӧʽ��
��3��Ϊ��ȷ�ⶨ�缫������ͭ���������������ʵ�鲽����Ⱥ�˳��Ӧ��
�ٳ������ǰ�缫����
�ڹ��µ���缫�ϵ�ͭ����ϴ
��������ˮ��ϴ����缫
�ܵ��º�ɵ缫�����
�ݵ��º�ɹ��µ�ͭ�������
���ٴε��º�ɺ����������
��4����֪���ӵĵ���Ϊ1.6��10-19C�����г������ӵ������ļ������ʽ��NA=
���㣺���ԭ��
ר�⣺�绯ѧר��
��������1������װ��ͼ������֪U�ι�ͨ��G�е�����������������B�缫Ϊ������AΪ��������·����Ӧ��ѭ�����ƴ�������Դ�����ӵ�������������������������U�ι�A�缫��B�缫�ӵ�Դ��������
��2�������ж�BΪ������������Һ�е�������ʧ���ӷ���������Ӧ����������G�Թ�����ͨ��������������͵绯��Ӧ���ɵⵥ�ʣ��������۱�����
��3�����ݵ缫������ͭ�������Լ��缫�ϲ��������������ⶨͭ�����ԭ������������Ҫ�������ǰ��缫��������
��4���ڵ���ǿ��ΪI A��ͨ��ʱ��Ϊt s��ȷ�����缫��������ͭ������Ϊm g�����ݵ���غ���ʽ����õ���
��2�������ж�BΪ������������Һ�е�������ʧ���ӷ���������Ӧ����������G�Թ�����ͨ��������������͵绯��Ӧ���ɵⵥ�ʣ��������۱�����
��3�����ݵ缫������ͭ�������Լ��缫�ϲ��������������ⶨͭ�����ԭ������������Ҫ�������ǰ��缫��������
��4���ڵ���ǿ��ΪI A��ͨ��ʱ��Ϊt s��ȷ�����缫��������ͭ������Ϊm g�����ݵ���غ���ʽ����õ���
���
�⣺��1������װ��ͼ������֪U�ι�ͨ��G�е�����������������B�缫Ϊ������AΪ��������·����Ӧ��ѭ�����ƴ�������Դ�����ӵ�������������������������U�ι�A�缫��B�缫�ӵ�Դ��������������·����˳����E-D-C-A-B-F��
�ʴ�Ϊ��A��B��
��2��BΪ��������������Һ�е�������ʧ���ӷ���������Ӧ�����������缫��ӦΪ��2Cl--2e-�TCl2����G�Թ�����ͨ��������������͵绯��Ӧ���ɵⵥ�ʣ��������۱�������Ӧ�����ӷ���ʽΪ��Cl2+2I-�TI2+2Cl-��
�ʴ�Ϊ��2Cl--2e-�TCl2��������ɫ��Cl2+2I-�TI2+2Cl-��
��3�����ݵ缫������ͭ�������Լ��缫�ϲ��������������ⶨͭ�����ԭ������������Ҫ�������ǰ��缫��������ʵ��֮ǰӦ�������ǰ�缫�����������缫�ں�ɳ���ǰ������������ˮ��ϴ���������缫�ں�ɳ��صIJ����б��밴�����-����-�ٺ��-�ٳ��ء����У���ֹCu�����������п������ڵ�����£���ɵ缫������õ��º�ɵķ�������ֹCu��������Ϊ��ȷ�ⶨ�缫������ͭ���������������ʵ�鲽����Ⱥ�˳��Ӧ�ǣ�
�ٳ������ǰ�缫������ʵ��֮ǰӦ�������ǰ�缫��������
��������ˮ��ϴ����缫��
�ܵ��º�ɵ缫�������
���ٴε��º�ɺ���������أ�
���Тڹ��µ���缫�ϵ�ͭ����ϴ���ݵ��º�ɹ��µ�ͭ��������������й��³���������ϴ�������Բ����в�ѡ��
�ʴ�Ϊ���٢ۢܢޣ�
��4�����ݵ���غ�õ���It=
��2NA��1.6��10-19��
����NA=
��
��
�ʴ�Ϊ��
��
��
�ʴ�Ϊ��A��B��
��2��BΪ��������������Һ�е�������ʧ���ӷ���������Ӧ�����������缫��ӦΪ��2Cl--2e-�TCl2����G�Թ�����ͨ��������������͵绯��Ӧ���ɵⵥ�ʣ��������۱�������Ӧ�����ӷ���ʽΪ��Cl2+2I-�TI2+2Cl-��
�ʴ�Ϊ��2Cl--2e-�TCl2��������ɫ��Cl2+2I-�TI2+2Cl-��
��3�����ݵ缫������ͭ�������Լ��缫�ϲ��������������ⶨͭ�����ԭ������������Ҫ�������ǰ��缫��������ʵ��֮ǰӦ�������ǰ�缫�����������缫�ں�ɳ���ǰ������������ˮ��ϴ���������缫�ں�ɳ��صIJ����б��밴�����-����-�ٺ��-�ٳ��ء����У���ֹCu�����������п������ڵ�����£���ɵ缫������õ��º�ɵķ�������ֹCu��������Ϊ��ȷ�ⶨ�缫������ͭ���������������ʵ�鲽����Ⱥ�˳��Ӧ�ǣ�
�ٳ������ǰ�缫������ʵ��֮ǰӦ�������ǰ�缫��������
��������ˮ��ϴ����缫��
�ܵ��º�ɵ缫�������
���ٴε��º�ɺ���������أ�
���Тڹ��µ���缫�ϵ�ͭ����ϴ���ݵ��º�ɹ��µ�ͭ��������������й��³���������ϴ�������Բ����в�ѡ��
�ʴ�Ϊ���٢ۢܢޣ�
��4�����ݵ���غ�õ���It=
| m |
| 64 |
����NA=
| 64 |
| m |
| It |
| 2��1.6��10-19 |
�ʴ�Ϊ��
| 64 |
| m |
| It |
| 2��1.6��10-19 |
���������⿼���˵��ԭ���ķ����жϣ���·���ӵķ����������غ�ļ���Ӧ�ã��缫��Ӧ���缫��Ӧ�������Ӧ�ã�ʵ��ⶨ����ͭ���������裬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
�����Ŀ
��֪ij��Һ�д��ڽ϶��H+��NO3-�������Һ�л����ܴ������ڵ��������ǣ�������
| A��Mg2+��Fe2+��SO42- |
| B��Ba2+��NH4+��Br- |
| C��Al3+��HCO3-��Cl- |
| D��Na+��SiO32-��ClO- |
X��Y��Z��M��WΪ���ֶ�����Ԫ�أ�X��Y��Z��ԭ���������ε�����ͬ����Ԫ�أ�������������֮��Ϊ15��X��Z���γ�XZ2���ӣ�Y��M�γɵ���̬�������ڱ�״���µ��ܶ�Ϊ0.76g?L-1��W����������X��Y��Z��M����Ԫ��������֮�͵�
������˵����ȷ���ǣ�������
| 1 |
| 2 |
| A��ԭ�Ӱ뾶��W��Z��Y��X��M |
| B��������Y2M4��M2Z2��ֻ���м��Լ� |
| C������������Ӧˮ��������ԣ�Y��X |
| D����X��Y��Z��M����Ԫ���γɵĻ�����һ���������Ӽ������й��ۼ� |
NA��ʾ�����ӵ�����������˵������ȷ���ǣ�������
| A��6.89���ڵ�KHSO4�к���0.1NA�������� |
| B��46 g NO2��N2O4��������к���ԭ������Ϊ3NA |
| C����2H��18O����ɵ�ˮ11g������������������Ϊ4NA |
| D����״���£�2.24L Cl2ͨ������H2O��NaOH��Һ��ת�Ƶĵ�������Ϊ0.1NA |
��NA��ʾ����٤��������ֵ������˵����ȷ���ǣ�������
| A��25�棬1.01��105Pa��64g SO2�к��еķ�����Ϊ2NA | ||
| B�����³�ѹ�£�11.2L Cl2���еķ�����Ϊ0.5NA | ||
| C����״���£�11.2L N2��CO�Ļ�����������Ϊ28g | ||
D��1mol N
|

 ���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һc��A��B������缫�壬ͨ��������ֱ����Դ��������ش��������⣺
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һc��A��B������缫�壬ͨ��������ֱ����Դ��������ش��������⣺