��Ŀ����
6��������أ�K2FeO4����һ�����͡���Ч�����ˮ���������Ҳ�����ɶ�����Ⱦ����֪���������ڵ��¼��Ի������ȶ���������ˮ����������ˮ�Ҵ����л��ܼ�������������ص��Ʊ��������£�
| �Ʊ����� | �������� |
| �ɷ� | Fe2O3��KNO3��KOH��ϼ��ȹ��������Ϻ�ɫ������غ�KNO2�Ȳ��� |
| ʪ�� | ǿ���Խ����У�Fe��NO3��3��KClO��Ӧ�����Ϻ�ɫ���������Һ |
| ��ⷨ | ���ŨNaOH��Һ�Ʊ�Na2FeO4 |
��2��ijʪ���Ʊ�������صĻ������̼��������£�

�ٿ��Ʒ�Ӧ�¶�Ϊ25�棬����1.5h���������ȹ�����Һ��Ϊ�Ϻ�ɫ���÷�Ӧ�����ӷ���ʽΪ3ClO-+2Fe3++10OH-�T2FeO42-+3Cl-+5H2O��
�����Ϻ�ɫ��Һ�м��뱥��KOH��Һ�������Ϻ�ɫ���壬���ˣ��õ�K2FeO4�ֲ�Ʒ�����������м��뱥��KOH��Һ�õ������ԭ���Ǹ��¶��¸�����ص��ܽ�ȱȸ������Ƶ��ܽ��С��
��K2FeO4�ֲ�Ʒ����Fe��OH��3��KCl�����ʣ����ؽᾧ�������з����ᴿ�����ᴿ����Ϊ����һ������K2FeO4�ֲ�Ʒ�������3mol/LKOH��Һ�У����ˣ�����Һ���ڱ�ˮԡ�У�����Һ�м��뱥��KOH��Һ�����衢���á����ˣ����Ҵ�ϴ��2��3�Σ�����ո������и��
������FeCl3 ����Fe��NO3��3����Դ��K2FeO4�IJ��ʺʹ��ȶ��ή�ͣ�һ��ԭ�����ڷ�Ӧ�¶Ⱥ�ǿ�����NaCl���ܽ�ȱ�NaNO3��ʹ��NaCl�ᾧȥ���ʽϵͣ���һ��ԭ����Cl-��FeO42-���������IJ�Ʒʹ���ʽ��ͣ�
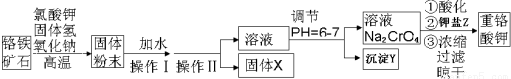
��3����ҵ�ϻ�����ͨ�����ŨNaOH��Һ�Ʊ�Na2FeO4���乤��ԭ������ͼ��ʾ�������ĵ缫��ӦΪFe-6e-+8OH-=FeO42-+4H2O�����п�ѭ��ʹ�õ����ʵĵ���ʽ��
 ��
��
��4����֪25��ʱ��Ksp[Fe��OH��3]=4.0��10-38��Ksp[Cu��OH��2]=2.2��10-20������¶��º���Fe3+��Cu2+Ũ�Ⱦ�Ϊ0.01mol/L����Һ�У��μ�ŨNaOH��Һ������ҺpH=10ʱ����Һ��c��Cu2+����c��Fe3+��=5.5��1013��1��
���� ��1��������ΪKNO3������ԭ����KNO2����ԭ��ΪFe2O3������������K2FeO4�����ݵ���ת���غ���㣻
��2�������������ڼ��������±�����������������Na2FeO4�����˳�ȥ����NaCl�������ƣ����뱥��KOH��Һ��Na2FeO4ת��Ϊ�ܽ�ȸ�С��K2FeO4����Ӧ����ʽΪNa2FeO4+2KOH=K2FeO4+2NaOH����ȴ�ᾧ�����ˣ�ϴȥ������ؾ�������KOH���������ʣ��õ�����Ʒ������أ�
�ٷ�Ӧ����ʽΪ��2Fe��NO3��3+3NaClO+10NaOH=2Na2FeO4+6NaNO3+3NaCl+5H2O��
��Na2FeO4ת��Ϊ�ܽ�ȸ�С��K2FeO4��������
���ɲ������̿�֪�����ؽᾧ�����ᴿ���ܽ����˳�ȥ��������������������������������أ����Ҵ�ϴ�ӣ�ϴȥ�������ӻ������������ܽ�µ���ʧ��
��FeO42-����ǿ�����ԣ���������Cl-��
��3��������Feʧȥʧȥ���ӣ���������������FeO42-��
�����ҵõ�A��ҺΪNaOH��Һ������ѭ�����ã�
��4��c��Cu2+��=$\frac{Ksp[Cu��OH��_{2}]}{{c}^{2}��O{H}^{-}��}$��c��Fe3+��=$\frac{Ksp[Fe��OH��_{3}]}{{c}^{3}��O{H}^{-}��}$���������㣮
��� �⣺��1��������ΪKNO3������ԭ����KNO2����ԭ��ΪFe2O3������������K2FeO4�����ݵ���ת���غ㣬��5-3����n��KNO3��=2��n��Fe2O3������6-3�����ɵã�n��KNO3����n��Fe2O3��=3��1��
�ʴ�Ϊ��3��1��
��2�������������ڼ��������±�����������������Na2FeO4�����˳�ȥ����NaCl�������ƣ����뱥��KOH��Һ��Na2FeO4ת��Ϊ�ܽ�ȸ�С��K2FeO4����Ӧ����ʽΪNa2FeO4+2KOH=K2FeO4+2NaOH����ȴ�ᾧ�����ˣ�ϴȥ������ؾ�������KOH���������ʣ��õ�����Ʒ������أ�
�ٷ�Ӧ����ʽΪ��2Fe��NO3��3+3NaClO+10NaOH=2Na2FeO4+6NaNO3+3NaCl+5H2O����Ӧ���ӷ���ʽΪ��3ClO-+2Fe3++10OH-�T2FeO42-+3Cl-+5H2O��
�ʴ�Ϊ��3ClO-+2Fe3++10OH-�T2FeO42-+3Cl-+5H2O��
�ڸ��¶��¸�����ص��ܽ�ȱȸ������Ƶ��ܽ��С�����뱥��KOH��Һ��������K+��Ũ�ȣ���С������ص��ܽ⣬�ٽ�������ؾ���������
�ʴ�Ϊ�����¶��¸�����ص��ܽ�ȱȸ������Ƶ��ܽ��С��
���ɲ������̿�֪�����ؽᾧ�����ᴿ���ܽ����˳�ȥ��������������������������������أ����Ҵ�ϴ�ӣ�ϴȥ�������ӻ������������ܽ�µ���ʧ��
�ʴ�Ϊ���ؽᾧ�����ˣ��Ҵ���
��FeO42-����ǿ�����ԣ�Cl-��FeO42-���������IJ�Ʒʹ���ʽ��ͣ�
�ʴ�Ϊ��Cl-��FeO42-���������IJ�Ʒʹ���ʽ��ͣ�
��3��������Feʧȥʧȥ���ӣ���������������FeO42-�������缫��ӦʽΪ��Fe-6e-+8OH-=FeO42-+4H2O��
�����ҵõ�A��ҺΪNaOH��Һ������ѭ�����ã�NaOH�ĵ���ʽΪ�� ��
��
�ʴ�Ϊ��Fe-6e-+8OH-=FeO42-+4H2O�� ��
��
��4����Һ��c��OH-��=10-4 mol/L��c��Cu2+��=$\frac{Ksp[Cu��OH��_{2}]}{{c}^{2}��O{H}^{-}��}$��c��Fe3+��=$\frac{Ksp[Fe��OH��_{3}]}{{c}^{3}��O{H}^{-}��}$���ɵ���Һ��c��Cu2+����c��Fe3+��=$\frac{2.2��1{0}^{-20}}{��1{0}^{-4}��^{2}}$��$\frac{4��1{0}^{-38}}{��1{0}^{-4}��^{3}}$=5.5��1013��1��
�ʴ�Ϊ��5.5��1013��1��
���� ���⿼��������ԭ��Ӧ���������������̡����ԭ�����ܶȻ��йؼ���ȣ��Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ�����

 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д� ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д�| A�� | �����Ը��������Һ�����������ϩ | |
| B�� | �÷�Һ©��������ȩ��ˮ | |
| C�� | �ñ�����ˮ�����ۻ����ȡ�屽 | |
| D�� | ����ɫʯ����Һ���鱽�������� |
| A�� | ������������Ȼ�л��߷��ӻ����û�е����ʾ�û������ | |
| B�� | HCHO��Һ��NH4��2SO4��Һ����ʹ�����ʱ��� | |
| C�� | ijЩ�����ʸ�Ũ�������û��� | |
| D�� | ���Բ��ö���������������ķ������롢�ᴿ������ |
 ��CO2��H2Ϊԭ����ȡ�Ҵ��ķ�ӦΪ��2CO2��g��+6H2��g���TCH3CH2OH��g��+3H2O��g����H��0��ijѹǿ�µ��ܱ������У���CO2��H2�����ʵ�����Ϊ1��3Ͷ�ϣ���ͬ�¶��£��ﵽƽ���ƽ����ϵ�и����ʵ����ʵ���������y%�����¶ȱ仯��ͼ��ʾ������˵����ȷ���ǣ�������
��CO2��H2Ϊԭ����ȡ�Ҵ��ķ�ӦΪ��2CO2��g��+6H2��g���TCH3CH2OH��g��+3H2O��g����H��0��ijѹǿ�µ��ܱ������У���CO2��H2�����ʵ�����Ϊ1��3Ͷ�ϣ���ͬ�¶��£��ﵽƽ���ƽ����ϵ�и����ʵ����ʵ���������y%�����¶ȱ仯��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | a���ƽ�ⳣ��С��b�� | B�� | b�㣺v����CO2��=v����H2O�� | ||
| C�� | a�㣺n��H2��=n��H2O�� | D�� | �¶Ȳ��䣬�������H2��D��CO2������ |
| ���� | �״� | ������ | ��������� |
| �е�/�� | 64.7 | 249 | 199.6 |
| ��Է������� | 32 | 122 | 136 |

����ʾ������������ֲ�Ʒ�������������״������ᡢ�������ˮ�ȣ�
��1��������һ�����Һ��ʱ��������Ũ�����������Ũ�����ܶȽϴ������ڱ����ᡢ�״���Ϸų������������״��ӷ�������Ӧ����ˮ��������ͬλ��18O��д���ܱ�ʾ��Ӧǰ��18Oλ�õĻ�ѧ����ʽ
 ��
����2����Ӧ��CH3OHӦ�����������Ǹ÷�Ӧ�ǿ��淴Ӧ�����Ӽ״�������ʹƽ�������ƶ�����������߱������ת���ʣ�
��3����Һʱ�ϲ�Һ��ӷ�Һ©�����Ͽڳ������������ʱ�¶ȿ�����199.6�����ң�
��4����������ͼ�м���Na2CO3��������ͨ����Ӧ�������ᡢ�����ᣬ���ͱ�����������ܽ�ȣ�
��5��ͨ�����㣬����������IJ���Ϊ65%��
| A�� | CH3CH3+Cl2$\stackrel{����}{��}$CH3CH2Cl+HCl | |
| B�� | CH2=CH2+HBr��CH3CH2Br | |
| C�� |  | |
| D�� | CH3COOH+CH3CH2OH$��_{��}^{Ũ����}$CH3COOCH2CH3+H20 |
| A�� | NO | B�� | N2O3 | C�� | NO2 | D�� | N2O4 |