��Ŀ����
8��ij������ܺ���Na+��NH4+��Fe3+��Fe2+��Cl-��I-��CO32-��SO42-�еļ������ӣ�ȡ�����������ݸù��壬��������ʵ�飨�����������ˮ�⼰ˮ�ĵ��룩����1��һ�ݹ�������ˮ����������BaCl2��Һ���ó���4.46g
��2����һ�ݹ�������ˮ�������NaOH�����Ϻ��ּ��ȣ�����ʹʪ��ĺ�ɫʯ����ֽ����ɫ������0.672L����״���������õ�������������ϴ�ӡ����գ����ջ��1.6g��ɫ���壮����˵����ȷ���ǣ�������
| A�� | �ù�����һ������NH4+��Cl-��SO42-��Na+ | |
| B�� | ��������ʵ�飬��ȷ���ù�������Cl- | |
| C�� | �ù�����ֻ����NH4+��Fe3+��SO42-��Cl- | |
| D�� | �ù�����һ��û��I-��CO32- |
���� �ɣ�2����һ�ݹ��������NaOH�����Ϻ��ּ��ȣ�����ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬������0.672L��$\frac{0.672L}{22.4L/mol}$=0.03mol����״���������Ժ���NH4+����0.03mol���õ�������������ϴ�ӡ����գ����ջ��1.6g��ɫ���壬Ϊ��������n��Fe��=$\frac{1.6g}{160g/mol}$��2=0.02mol����Fe3+��Fe2+�е�һ�ֻ����֣���0.02mol����ԭ��Һ��һ������CO32-��
�ɣ�1��һ�ݹ�������ˮ����ɫ����Һ��˵������Cu2+����������BaCl2��Һ���ó���4.46g��һ������SO42-����n��SO42-��=n��BaSO4��=$\frac{4.66g}{233g/mol}$=0.02mol��
�Դ˽����⣮
��� �⣺A��������������֪��һ����NH4+��SO42-�����ʵ����ֱ�Ϊ0.03mol��0.02mol����Fe3+��Fe2+�е�һ�ֻ����֣���0.02mol���ɵ���غ��֪һ������һ�����������ӣ���A����
B��������������֪������ȷ���ù�������Cl-����B��ȷ��
C���ù�����ܺ�������ֻ����NH4+��Fe2+��SO42-��I-��Cl-����C����
D��������Fe3+����ԭ�����к�I-����D����
��ѡB��
���� ���⿼��������ƶϣ�Ϊ��Ƶ���㣬��������֮��ķ�Ӧ�����ӹ��桢����غ�Ϊ���Ĺؼ������ط������ƶ������Ŀ��飬ע����ܺ������ӣ���Ŀ�ѶȲ���

| X | |||
| W | Y | Z |
| A�� | X��Y��������Ԫ���γɵĻ������п��ܼ������Ӽ������й��ۼ� | |
| B�� | Y���������ͨ�����BaCl2��Һ��һ���������ɫ���� | |
| C�� | WԪ�صĵ��������õİ뵼����ϣ�����ZԪ�ؿ��γɻ�����WZ4 | |
| D�� | ����W3X4�У�ÿ��ԭ���������ﵽ8�����ȶ��ṹ |
| ѡ�� | ʵ����� | ���� | ���ͻ���� |
| A | �ò�˿պȡ��ҺX������ɫ��Ӧʵ�� | ����ʻ�ɫ | ��ҺXһ����������Һ |
| B | ��Cl2ͨ��ʯ����Һ�� | ��Һ�ȱ�����ɫ | Cl2����Ư���� |
| C | ��NaHCO3��Һ�еμ�NaAlO2��Һ | �а�ɫ������������� | AlO2-��HCO3-������˫ˮ�ⷴӦ |
| D | ��FeBr2��Һ�м���������ˮ���ټ� CCl4�� | CCl4����ɫ | Fe2+�Ļ�ԭ��ǿ��Br- |
| A�� | A | B�� | B | C�� | C | D�� | D |
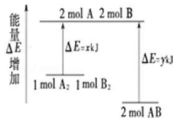
| A�� | �÷�Ӧ�����ȷ�Ӧ | |
| B�� | ���� 1molA-A ���� 1mol B-B ���ų� xkJ ���� | |
| C�� | ���� 2molA-B ����Ҫ���� y kJ ������ | |
| D�� | 1molA2�� 1molB2 ������������ 2molAB �������� |

 ��ľ����Ҫ����K2CO3��������K2SO4��KCl��ij��ѧ��ȤС����Ӳ�ľ������ȡ�����ξ��壬���ⶨK2CO3�ĺ�����
��ľ����Ҫ����K2CO3��������K2SO4��KCl��ij��ѧ��ȤС����Ӳ�ľ������ȡ�����ξ��壬���ⶨK2CO3�ĺ�����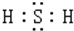 ��H2S��ˮ��Һ�ڿ����з���ʱ��������ǣ���˵����H2S��ǿ�Ļ�ԭ�ԣ�
��H2S��ˮ��Һ�ڿ����з���ʱ��������ǣ���˵����H2S��ǿ�Ļ�ԭ�ԣ�