��Ŀ����
 �ѱ���Ϊ��δ�����͵Ľ��������Իش��������⣺
�ѱ���Ϊ��δ�����͵Ľ��������Իش��������⣺��1��TiԪ�ػ�̬ԭ�ӵļ۵��Ӳ��Ų�ʽΪ
��2����Ti�Ļ������У����Գ���+2��+3��+4���ֻ��ϼۣ�������+4�۵�Ti��Ϊ�ȶ���ƫ���ᱵ�����ȶ��Ժã���糣���ߣ���С�ͱ�ѹ������Ͳ���������ж���Ӧ�ã�ƫ���ᱵ�����о����Ľṹʾ��ͼ���£������Ļ�ѧʽ��
��3������Ti3+�������[TiCl��H2O��5]Cl2?H2O����������������
��4����֪������ABn�ͷ��ӣ�A������ԭ�ӣ�B����λԭ�ӣ������ṹ����ʱ�������ڵļ��Ǵ�Сȡ��������ԭ�ӻ���λԭ�ӵĵ縺�Դ�С������ԭ����ͬʱ����λԭ�ӵĵ縺��Խ����ԽС����λԭ����ͬʱ������ԭ�ӵĵ縺��Խ����Խ����NF3��PF3��NH3�ļ����ɴ�С��˳��Ϊ
��5���Ҷ�����H2N-CH2-CH2-NH2�������е�ԭ�ӹ�����ӻ�����Ϊ���Ҷ��������װ�[N��CH3��3]�����ڰ������Ҷ��������װ��ķе�ߵĶ࣬ԭ��
���㣺�����ijɼ����,���ܡ����������Ǽ���Ӧ��,ԭ�ӹ���ӻ���ʽ���ӻ������ж�
ר�⣺��ѧ���뾧��ṹ
��������1����Ԫ��ԭ�Ӻ��������Ϊ22�����������ԭ����д��̬ԭ�ӵĵ����Ų�ʽ��������д�۵����Ų���
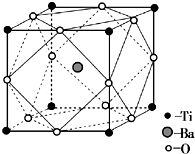
��2�����þ�̯�����㾧���и���ԭ�ӵ���Ŀ֮�ȣ��ݴ�ȷ����ѧʽ��������Tiλ�ڶ��㣬��֮�����Oԭ�Ӵ����������ϣ��붥��Tiԭ�ӳɶԳƽṹ��
��3�����������Ľṹ�ص����������е����弰��λ����
��4��NF3��PF3����λԭ�Ӷ���F��N�ĵ縺�Դ���P������NF3�ļ��Ǵ���PF3��NF3��NH3������ԭ����ͬ��F�ĵ縺�Դ���H������NF3�ļ���С��NH3��
��5���Ҷ�����H2N-CH2-CH2-NH2������֮������γ�����������װ�[N��CH3��3]����֮�䲻���γ������
��2�����þ�̯�����㾧���и���ԭ�ӵ���Ŀ֮�ȣ��ݴ�ȷ����ѧʽ��������Tiλ�ڶ��㣬��֮�����Oԭ�Ӵ����������ϣ��붥��Tiԭ�ӳɶԳƽṹ��
��3�����������Ľṹ�ص����������е����弰��λ����
��4��NF3��PF3����λԭ�Ӷ���F��N�ĵ縺�Դ���P������NF3�ļ��Ǵ���PF3��NF3��NH3������ԭ����ͬ��F�ĵ縺�Դ���H������NF3�ļ���С��NH3��
��5���Ҷ�����H2N-CH2-CH2-NH2������֮������γ�����������װ�[N��CH3��3]����֮�䲻���γ������
���
�⣺��1����Ԫ�ص�ԭ������Ϊ22����̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63S23p63d24s2����[Ar]3d24s2����22TiԪ�ػ�̬ԭ�ӵļ۵��Ӳ��Ų�ʽΪ3d24s2��
�ʴ�Ϊ��3d24s2��
��2��������Ba��Ti��Oԭ�ӵ���Ŀ֮��=1����8��
������12��
��=1��1��3�������ʽΪBaTiO3��������Tiλ�ڶ��㣬��֮�����Oԭ�Ӵ����������ϣ��붥��Tiԭ�ӳɶԳƽṹ���ʹʾ�����ÿ��Ti��Χ��6��Oԭ�ӣ�
�ʴ�Ϊ��BaTiO3��6��
��3�������[TiCl��H2O��5]Cl2?H2O����Ԫ�ص���λ����H2O��Cl-����λ��Ϊ5+1=6���ʴ�Ϊ��H2O��Cl-��6��
��4��NF3��PF3����λԭ�Ӷ���F��N�ĵ縺�Դ���P������NF3�ļ��Ǵ���PF3��NF3��NH3������ԭ����ͬ��F�ĵ縺�Դ���H������NF3�ļ���С��NH3����˼���NH3��NF3��PF3���ʴ�Ϊ��NH3��NF3��PF3��
��5���Ҷ�����H2N-CH2-CH2-NH2������֮������γ���������װ�[N��CH3��3]����֮�䲻���γ���������Ҷ����ķе�ϸߣ�
�ʴ�Ϊ���Ҷ�������֮������γ���������װ�����֮�䲻���γ������
�ʴ�Ϊ��3d24s2��
��2��������Ba��Ti��Oԭ�ӵ���Ŀ֮��=1����8��
| 1 |
| 8 |
| 1 |
| 2 |
�ʴ�Ϊ��BaTiO3��6��
��3�������[TiCl��H2O��5]Cl2?H2O����Ԫ�ص���λ����H2O��Cl-����λ��Ϊ5+1=6���ʴ�Ϊ��H2O��Cl-��6��
��4��NF3��PF3����λԭ�Ӷ���F��N�ĵ縺�Դ���P������NF3�ļ��Ǵ���PF3��NF3��NH3������ԭ����ͬ��F�ĵ縺�Դ���H������NF3�ļ���С��NH3����˼���NH3��NF3��PF3���ʴ�Ϊ��NH3��NF3��PF3��
��5���Ҷ�����H2N-CH2-CH2-NH2������֮������γ���������װ�[N��CH3��3]����֮�䲻���γ���������Ҷ����ķе�ϸߣ�
�ʴ�Ϊ���Ҷ�������֮������γ���������װ�����֮�䲻���γ������
���������⿼���������Ų����ɡ������ļ��㡢���ӿռ�ṹ������뻯ѧ����֪ʶ���ۺ��Խϴ��Ѷ��еȣ��Ƕ�֪ʶ���ۺ����ã���Ҫѧ���߱���ʵ�Ļ�������������������������

��ϰ��ϵ�д�
 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�
�����Ŀ
P�����������״��ȷ���ǣ�������
| A�����ζԳ� |
| B���Զ��Գ� |
| C������ֵ��x��y��z���ϵķĴ��� |
| D�����ഹֱ�Ļ����� |
������Һ�и�����Ũ�ȹ�ϵһ����ȷ���ǣ�������
| A��ij������Һ��ֻ��NH4+��Cl-��H+��OH-�������ӣ���Һ��һ�����ڣ�c��Cl-����c��NH4+����c��H+����c��OH-�� |
| B�����ʵ���Ũ����ͬ��4����Һ����CH3COONa����NaNO3����Na2CO3����NaOH��pH�Ĵ�С˳���ǣ��ܣ��ۣ��٣��� |
| C����Na2CO3��NaHCO3�Ļ����Һ�У�c��Na+��+c��H+��=c��HCO3-��+c��OH-��+c��CO32-�� |
| D��25��ʱ��pH=10��CH3COONa��Һ��pH=10�İ�ˮ�У���ˮ�������c��OH-��֮��Ϊ1��1 |
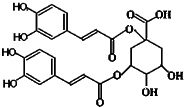 ������һ���½ṹ���͵Ŀ������ײ����Ϳ����̲������Ļ������ṹ��ͼ��ʾ���й����ص�˵����ȷ���ǣ����ĸ���ͬ���ŵ�̼��������̼����������
������һ���½ṹ���͵Ŀ������ײ����Ϳ����̲������Ļ������ṹ��ͼ��ʾ���й����ص�˵����ȷ���ǣ����ĸ���ͬ���ŵ�̼��������̼����������| A�������к���6������̼ԭ�� |
| B��һ���������ܷ���������Ӧ����ȥ��Ӧ |
| C���������Ȼ�����Һ������ɫ��Ӧ |
| D��1 mol����������11 mol NaOH��Ӧ |
����������ȷ���ǣ�������
| A��0.1mol/L NaHCO3��Һ�У�c��H+��+c��Na+��=c��OH-��+c��HCO3-��+c��CO32-�� |
B��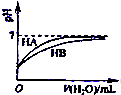 ��ʾ�����£�ϡ��HA��HB�������ϡ��Һʱ����ҺPH���ˮ���ı仯����NaA��Һ��PH����ͬŨ�ȵ�NaB��Һ��PH |
C�� ��ʾ������������ͬʱ���ֱ���T1��T2�¶�����CO2��H2�ϳɼ״������ʵ�����ʱ��仯�������CO2��H2�ϳɼ״��Ƿ��ȷ�Ӧ |
D�� ��ʾ��1.000 mol/L��ˮ����20.00mL1.000 mol/L�����У���ҺPH���¶�����백ˮ����仯���� |
ij��ɫ��Һ��ֻ���ܺ��Т�Na+����Ba2+����Clһ����Iһ����SO32һ����SO42һ �����е������֣�����ˮ�������H+��OHһ�������ν�������ʵ�飬��ÿ�������Լ����������۲쵽�������±������н�����ȷ���ǣ�������
| ���� | ���� | ���� |
| �� | ��pH��ֽ���� | ��Һ��pH����7 |
| �� | ����Һ�еμ���ˮ���ټ���CCl4������ | CCl4����Ϻ�ɫ |
| �� | ������ˮ��Һ�м���Ba��NO3��2��Һ��ϡHNO3 | �а�ɫ�������� |
| �� | ���ˣ�����Һ�м���AgNO3��Һ��ϡHNO3 | �а�ɫ�������� |
| A���϶����е������Ǣۢܢ� |
| B���϶�û�е������Ǣڢ� |
| C������ȷ���������Ǣۢ� |
| D�����ܺ��е������Ǣ٢� |
 һ���л���Ļ�ѧʽΪC4H4�����ӽṹ��ͼ��ʾ�������л���������������Ϻ���գ����ɵ�±����������У�������
һ���л���Ļ�ѧʽΪC4H4�����ӽṹ��ͼ��ʾ�������л���������������Ϻ���գ����ɵ�±����������У�������| A��2 | B��4 | C��5 | D��6 |
�ȡ��塢���±��Ԫ����Ҫ���Ժ�ˮ���ܶຣ��ֲ���ж����д����ĵ⣬��Ӧ���ǴӺ��������ȡ�����Ҫ��Ӧ����Ӧ���Ǵ�������ʯ����ȡ�����Ҫ��Ӧ��2NaI+MnO2+3H2SO4�T2NaHSO4+MnSO4+2H2O+I2 ��
2NaIO3+5NaHSO3�T2Na2SO4+3NaHSO4+H2O+I2�������й�˵����ȷ���ǣ�������
2NaIO3+5NaHSO3�T2Na2SO4+3NaHSO4+H2O+I2�������й�˵����ȷ���ǣ�������
| A��NaI��NaIO3��һ���������ܷ�Ӧ����I2 |
| B��I2�ڷ�Ӧ�����ǻ�ԭ����ڷ�Ӧ�������������� |
| C��������Ӧ�����ɵ�����I2ʱת�Ƶ�������� |
| D�������ԣ�Mn02��SO42-��I03-��I2 |