��Ŀ����
����ʯ�ǹ�ҵ��������Ҫԭ��֮һ������Ҫ�ɷ�Ϊ����������������в�����Ԫ�غ���Ԫ�أ������ʲ������ᷴӦ����ij�о���ѧϰС���ij����ʯ������������Ļ�ѧʽ����̽����
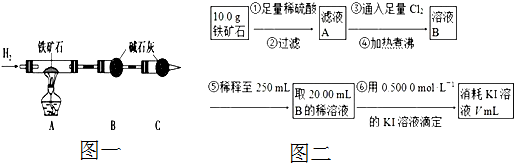
������ʯ�к������IJⶨ������ʵ����̲��������벹������
��1������ͼһ��װ�����������װ�õ������ԣ�
��2����8.0g����ʯ����Ӳ�ʲ������У�װ��B��C�е�ҩƷ��ͼ��ʾ���г�������ʡ�ԣ���
��3������˵����ܿڴ����ϵػ���ͨ��H2�� ���ٵ�ȼA���ƾ��ƣ�
��4����ַ�Ӧ�����ƾ��ƣ��ٳ���ͨ��������Ӳ�ʲ�������ȫ��ȴ��
��5����÷�Ӧ��װ��B����2.25g��������ʯ�����İٷֺ���Ϊ ��
������ʯ�к������IJⶨ��������ͼ����
��1����������������� ��
��2����������õ��IJ����������ձ�����ͷ�ιܡ��������� ��
��3�������йز���IJ�����˵����ȷ���� ��
a����Ϊ��ˮΪ��ɫ�����Եζ������в����ָʾ��
b����ƿ����Ҫ�ô���Һ��ϴ
c���ζ�ǰû�����ݣ��ζ������ζ��ܼ��첿�������ݣ���ⶨ���ƫ��
d���ζ������У��۾�ע�ӵζ�����Һ��仯
e���ζ�������30s����Һ���ָ�ԭ������ɫ���ٶ���
��4���ζ�������������0.5000mol?L-1 KI��Һ20.00mL��
���ɢ���������������ʯ����ֻ��һ��������������仯ѧʽΪ ��

������ʯ�к������IJⶨ������ʵ����̲��������벹������
��1������ͼһ��װ�����������װ�õ������ԣ�
��2����8.0g����ʯ����Ӳ�ʲ������У�װ��B��C�е�ҩƷ��ͼ��ʾ���г�������ʡ�ԣ���
��3������˵����ܿڴ����ϵػ���ͨ��H2��
��4����ַ�Ӧ�����ƾ��ƣ��ٳ���ͨ��������Ӳ�ʲ�������ȫ��ȴ��
��5����÷�Ӧ��װ��B����2.25g��������ʯ�����İٷֺ���Ϊ
������ʯ�к������IJⶨ��������ͼ����
��1�����������������
��2����������õ��IJ����������ձ�����ͷ�ιܡ���������
��3�������йز���IJ�����˵����ȷ����
a����Ϊ��ˮΪ��ɫ�����Եζ������в����ָʾ��
b����ƿ����Ҫ�ô���Һ��ϴ
c���ζ�ǰû�����ݣ��ζ������ζ��ܼ��첿�������ݣ���ⶨ���ƫ��
d���ζ������У��۾�ע�ӵζ�����Һ��仯
e���ζ�������30s����Һ���ָ�ԭ������ɫ���ٶ���
��4���ζ�������������0.5000mol?L-1 KI��Һ20.00mL��
���ɢ���������������ʯ����ֻ��һ��������������仯ѧʽΪ
���㣺̽�����ʵ���ɻ�������ʵĺ���
ר�⣺ʵ��̽�������ݴ�����
��������3����������ȼ��ʱ�ɷ�����ը����˵�ȼ�ƾ���ǰҪ����Cװ�ó��ڴ������Ĵ��ȣ�
��5����Ӧ��װ��B����2.25g������������������Ӧ���������������ֵ�����Ը��ݲ����������㣻
������ʯ����������ϡ�����ܽ⣬���˳�ȥ���ʣ��õ�ֻ���������ӵ���Һ����ͨ��������Һ�е������������������������ӣ����õ⻯�����ζ����ݴ˷������㣻
��1����п��Խ�ˮ�е�������ܣ�
��2������ϡ��Һ�������һ���������Һ��ѡ����������ش�
��3�����ݵζ������Լ��ζ������е�ʵ��������֪ʶ���ش��жϣ�
����װ��B����2.25gΪ����ˮ��������������Ԫ���غ�8.0g��ʯ�м���n��O�������в������2Fe3++2I-=2Fe2++I2����������KI��Һ�������n��Fe3+��������FeԪ���غ����10g��ʯ��n��Fe�����ݴ�ȷ�������ﻯѧʽ��
��5����Ӧ��װ��B����2.25g������������������Ӧ���������������ֵ�����Ը��ݲ����������㣻
������ʯ����������ϡ�����ܽ⣬���˳�ȥ���ʣ��õ�ֻ���������ӵ���Һ����ͨ��������Һ�е������������������������ӣ����õ⻯�����ζ����ݴ˷������㣻
��1����п��Խ�ˮ�е�������ܣ�
��2������ϡ��Һ�������һ���������Һ��ѡ����������ش�
��3�����ݵζ������Լ��ζ������е�ʵ��������֪ʶ���ش��жϣ�
����װ��B����2.25gΪ����ˮ��������������Ԫ���غ�8.0g��ʯ�м���n��O�������в������2Fe3++2I-=2Fe2++I2����������KI��Һ�������n��Fe3+��������FeԪ���غ����10g��ʯ��n��Fe�����ݴ�ȷ�������ﻯѧʽ��
���
�⣺��3����������ȼ��ʱ�ɷ�����ը����˵�ȼ�ƾ���ǰҪ����Cװ�ó��ڴ������Ĵ��ȣ�
�ʴ�Ϊ����Cװ�ó��ڴ������鴿��
��5��װ��B����2.25g�����ݷ�Ӧ��ʵ�ʣ����ӵ���ˮ��������������Ԫ�ص�����������
��100%=25%��
�ʴ�Ϊ��25%��
������ʯ����������ϡ�����ܽ⣬���˳�ȥ���ʣ��õ�ֻ���������ӵ���Һ����ͨ��������Һ�е������������������������ӣ����õ⻯�����ζ���
��1��������ʯ�м������ᣬ��Ӧ���������������Һ�������ڹ�����������Һ�������������������е������Ǹ�����Һ���ܽ�Ĺ�����Cl2���ʴ�Ϊ��������Һ���ܽ�Ĺ�����Cl2��
��2������ƿ��һ�ֶ�������������ϡ�͵�250mL������õ������У��ձ�������������ͷ�ιܡ�250mL����ƿ���ʴ�Ϊ��250mL����ƿ��
��3��a����ˮΪ��ɫ������������Ҳ�ǻ�ɫ��Һ���ζ����������ָʾ������a����
b����ƿ����Ҫ�ô���Һ��ϴ������Ũ��ƫ��b��ȷ��
c���ζ������ζ��ܼ��첿�������ݣ����¶���ƫ����ⶨ���ƫ��c����
d���ζ������У��۾�ע����ƿ����ɫ�ı仯����d����
e���ζ�������30s����Һ���ָ�ԭ������ɫ�ٶ�������e��ȷ��
�ʴ�Ϊ��be��
����װ��B����2.25gΪ����ˮ��������������Ԫ���غ㣬8.0g��ʯ��n��O��=n��H2O��=
=0.125mol��10g��ʯ��n��O��=0.125��
mol��
��������2Fe3++2I-=2Fe2++I2��n��Fe3+��=0.02L��0.5mol/L��
=0.125mol����10g��ʯ��n��Fe��=n��Fe3+��=0.125mol��
��n��Fe����n��O��=0.125mol��0.125��
mol=4��5������ѧʽΪ��Fe4O5��
�ʴ�Ϊ��Fe4O5��
�ʴ�Ϊ����Cװ�ó��ڴ������鴿��
��5��װ��B����2.25g�����ݷ�Ӧ��ʵ�ʣ����ӵ���ˮ��������������Ԫ�ص�����������
2.25g��
| ||
| 8.0 |
�ʴ�Ϊ��25%��
������ʯ����������ϡ�����ܽ⣬���˳�ȥ���ʣ��õ�ֻ���������ӵ���Һ����ͨ��������Һ�е������������������������ӣ����õ⻯�����ζ���
��1��������ʯ�м������ᣬ��Ӧ���������������Һ�������ڹ�����������Һ�������������������е������Ǹ�����Һ���ܽ�Ĺ�����Cl2���ʴ�Ϊ��������Һ���ܽ�Ĺ�����Cl2��
��2������ƿ��һ�ֶ�������������ϡ�͵�250mL������õ������У��ձ�������������ͷ�ιܡ�250mL����ƿ���ʴ�Ϊ��250mL����ƿ��
��3��a����ˮΪ��ɫ������������Ҳ�ǻ�ɫ��Һ���ζ����������ָʾ������a����
b����ƿ����Ҫ�ô���Һ��ϴ������Ũ��ƫ��b��ȷ��
c���ζ������ζ��ܼ��첿�������ݣ����¶���ƫ����ⶨ���ƫ��c����
d���ζ������У��۾�ע����ƿ����ɫ�ı仯����d����
e���ζ�������30s����Һ���ָ�ԭ������ɫ�ٶ�������e��ȷ��
�ʴ�Ϊ��be��
����װ��B����2.25gΪ����ˮ��������������Ԫ���غ㣬8.0g��ʯ��n��O��=n��H2O��=
| 2.25g |
| 18g/mol |
| 10 |
| 8 |
��������2Fe3++2I-=2Fe2++I2��n��Fe3+��=0.02L��0.5mol/L��
| 250mL |
| 20mL |
��n��Fe����n��O��=0.125mol��0.125��
| 10 |
| 8 |
�ʴ�Ϊ��Fe4O5��
���������⿼����̽������ʯ����Ԫ�غ���Ԫ�صĺ����ķ�������һ��̽�����ʵ���ɡ��������ʵĺ���֪ʶ��һ���ۺϿ����⣬����ѧ�������ͽ�������������Ϊ�߿��������ͣ��ۺ���ǿ���Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
�������ӷ���ʽ����д��ȷ���ǣ�������
| A������������ϡ���ᷴӦ��Fe+4H++NO3-=Fe3++2H2O+NO�� |
| B��������SO2����ͨ��NaClO��Һ�У�SO2+ClO-+H2O=SO32-+2HClO |
| C��������Һ�м�����������������Һ��Ba2++OH-+H++SO42-=BaSO4+H2O |
| D����Mg��OH��2��Һ�е���FeCl3��Һ��3 Mg��OH��2��s��+2 Fe3+?2 Fe��OH��3��s��+3Mg2+ |
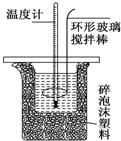 �ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��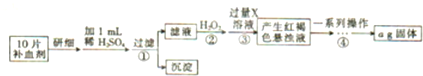
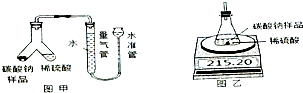 ij���������ð�������Ĵ����к��������Ȼ������ʣ�Ϊ�˲ⶨ�ò�Ʒ��̼���ƵĴ��ȣ�ij��ѧ�о���ѧϰС������йط���������ͼʵ�飺
ij���������ð�������Ĵ����к��������Ȼ������ʣ�Ϊ�˲ⶨ�ò�Ʒ��̼���ƵĴ��ȣ�ij��ѧ�о���ѧϰС������йط���������ͼʵ�飺