��Ŀ����
 ij���������ð�������Ĵ����к��������Ȼ������ʣ�Ϊ�˲ⶨ�ò�Ʒ��̼���ƵĴ��ȣ�ij��ѧ�о���ѧϰС������йط���������ͼʵ�飺
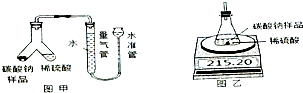
ij���������ð�������Ĵ����к��������Ȼ������ʣ�Ϊ�˲ⶨ�ò�Ʒ��̼���ƵĴ��ȣ�ij��ѧ�о���ѧϰС������йط���������ͼʵ�飺��1������һ��С�������ͼ����ʾʵ��װ�ã�Y����һ����м���̼������Ʒ����һ��������ϡ���ᣬ��ϡ������뵽������Ʒʹ֮��Ӧ�IJ���Ϊ
��2����һ��С�������ͼ����ʾװ�ã���ķ�Ӧǰ����й����������ȡ16.50g������Ʒ�����ձ��У����ձ����ڵ�����ƽ�ϣ��ٰ�150.00gϡ���ᣨ������������Ʒ�У�����װ����CO2���������۲�����仯�����ʾ��
| ʱ��/s | 0 | 5 | 10 | 15 |
| ����/g | 215.20 | 211.40 | 208.60 | 208.60 |
��ʵ���в�����CO2��������Ϊ
�ڸò�Ʒ��̼���Ƶ����������������ȷ��С�����һλ��
�۵���С����ƵIJ�ͬǰ���ֵķ����ⶨ̼���Ƶ��������������ܵķ�����
���㣺̽�����ʵ���ɻ�������ʵĺ���
ר�⣺ʵ��̽�������ݴ�����
��������1��Y����б��ʹҺ�嵹��̼������Ʒ�У�̼���ƺ����ᷴӦ�����Ȼ��ơ�������̼��ˮ���ռ���aL����Ϊ������̼����������ݷ�Ӧ����ʽ����̼���Ƶ�������
��2��������װ��ͼ������Ӧǰ��������СΪ������̼��������
�����ݻ�ѧ����ʽ��֪���ɶ�����̼���ʵ�����̼�������ʵ�����ͬ������õ�̼���Ƶ�����������������̼Я��ˮ�����ݳ�ʹ������С�Ķࣻ
��ǰ���ַ�����ͨ���ⶨ������������������������̼���Ƶ�����������Ҳ��ͨ������̼���γ������Ȼ����������ⶨ��
��2��������װ��ͼ������Ӧǰ��������СΪ������̼��������
�����ݻ�ѧ����ʽ��֪���ɶ�����̼���ʵ�����̼�������ʵ�����ͬ������õ�̼���Ƶ�����������������̼Я��ˮ�����ݳ�ʹ������С�Ķࣻ
��ǰ���ַ�����ͨ���ⶨ������������������������̼���Ƶ�����������Ҳ��ͨ������̼���γ������Ȼ����������ⶨ��
���
�⣺��1������ʾװ�ã�̼���ƺ�ϡ�����װ��Y�Ͳ������У�ʹ̼������Ʒ��ϡ���ᷴӦ�IJ���ΪҺ�嵹������з�Ӧ����Y����б��ʹ������Һ���뵽̼������Ʒ�У�������Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+H2SO4=Na2SO4+H2O+CO2�������ӷ���ʽΪ��CO32-+2H+=H2O+CO2 ����
Na2CO3+H2SO4=Na2SO4+H2O+CO2����
106g 22.4L
m aL
m=
=
g��
�ʴ�Ϊ����Y����б��ʹ������Һ���뵽̼������Ʒ�У�CO32-+2H+=H2O+CO2 ����
��
��2���ٷ�Ӧǰ��������СΪ������̼��������215.20-208.60=6.60g��
�ڶ�����̼���ʵ���=̼�������ʵ���=
=0.15mol��̼���Ƶ�����=0.15mol��106g/mol=15.9g��̼���Ƶ���������Ϊ��
��100%=96.4%��
������̼Я��ˮ�����ݳ�ʹ������С�Ķ࣬������̼����������̼������������ƫ��
��ǰ���ַ�����ͨ���ⶨ������������������������̼���Ƶ�����������Ҳ��ͨ������̼���γ������Ȼ����������ⶨ�������Ϊ��ȡһ��������Ʒ�������������Ȼ�����Һ�����ˡ�ϴ�ӡ����������������������������ȡһ��������Ʒ��������������������Һ���ټ�ϡ���ᣬ���ˡ�ϴ�ӡ������ò������������������ݳ�������������̼���ƻ��Ȼ��Ƶ���������������̼���Ƶ�����������
�ʴ�Ϊ��6.6g��96.4%���ݳ��Ķ�����̼�к���ˮ������ȡһ��������Ʒ�������������Ȼ�����Һ�����ˡ�ϴ�ӡ��������������������������ȡһ��������Ʒ��������������������Һ���ټ�ϡ���ᣬ���ˡ�ϴ�ӡ������ò���������������
Na2CO3+H2SO4=Na2SO4+H2O+CO2����
106g 22.4L
m aL
m=
| 106g��aL |
| 22.4L |
| 106a |
| 22.4 |
�ʴ�Ϊ����Y����б��ʹ������Һ���뵽̼������Ʒ�У�CO32-+2H+=H2O+CO2 ����
| 106a |
| 22.4 |
��2���ٷ�Ӧǰ��������СΪ������̼��������215.20-208.60=6.60g��
�ڶ�����̼���ʵ���=̼�������ʵ���=
| 6.60g |
| 44g/mol |
| 15.9g |
| 16.5g |
������̼Я��ˮ�����ݳ�ʹ������С�Ķ࣬������̼����������̼������������ƫ��
��ǰ���ַ�����ͨ���ⶨ������������������������̼���Ƶ�����������Ҳ��ͨ������̼���γ������Ȼ����������ⶨ�������Ϊ��ȡһ��������Ʒ�������������Ȼ�����Һ�����ˡ�ϴ�ӡ����������������������������ȡһ��������Ʒ��������������������Һ���ټ�ϡ���ᣬ���ˡ�ϴ�ӡ������ò������������������ݳ�������������̼���ƻ��Ȼ��Ƶ���������������̼���Ƶ�����������
�ʴ�Ϊ��6.6g��96.4%���ݳ��Ķ�����̼�к���ˮ������ȡһ��������Ʒ�������������Ȼ�����Һ�����ˡ�ϴ�ӡ��������������������������ȡһ��������Ʒ��������������������Һ���ټ�ϡ���ᣬ���ˡ�ϴ�ӡ������ò���������������
���������⿼�����������ʵ�ʵ��̽�������жϣ�ʵ����̵����ݴ����������������ʵķ���Ӧ���ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�
�����Ŀ
�ص�һ��ͬλ��
Ho����������ҽ�Ƽ���������ԭ�Ӻ�������������������֮���ǣ�������
165 67 |
| A��31 | B��67 | C��98 | D��165 |
 ������R1��R2Ϊδ֪���ֵĽṹ������֪X���Է�����ͼ��ʾ��ת����
������R1��R2Ϊδ֪���ֵĽṹ������֪X���Է�����ͼ��ʾ��ת����

 ����Һ��NaHCO3��Һ
����Һ��NaHCO3��Һ +2HCO3-�T
+2HCO3-�T +2CO2��+2H2O��
+2CO2��+2H2O��

