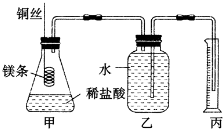
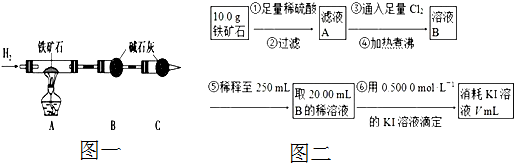
��Ŀ����
A�Ǵ��ߴ��ӹ�ʵ����ȡ��һ���в�ҩ��Ч�ɷ֣�����̼���⡢��Ԫ����ɵ�������B������Ƭ��������ҽҩ�����㾫�����Եȣ�C�ķ����к���4����ԭ�ӣ�D��ֻ��һ����ԭ�ӣ���Na��Ӧ�ų�H2��FΪ��������ϸ�Ķ�����ת����ϵ��M����ֵ��ʾ��Ӧ�л������Է������������ش��������⣺
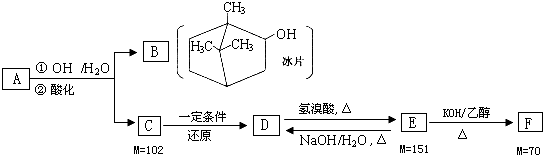
��1��B�ķ���ʽΪ ��F�ķ���ʽΪ ��
��2��д����������������C��ͬ���칹��Ľṹ��ʽ������������ ���ܷ���������Ӧ��
�� ����д���֣���
��3��д��D��E�Ļ�ѧ����ʽ�� ��

��1��B�ķ���ʽΪ
��2��д����������������C��ͬ���칹��Ľṹ��ʽ������������ ���ܷ���������Ӧ��
��3��д��D��E�Ļ�ѧ����ʽ��
���㣺�л�����ƶ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
������FΪ������Է�������Ϊ70���������Cԭ�������ĿΪ
=5��10����F�ķ���ʽ��C5H10����D��E��F��ת����֪��EΪ����������E��F����Է���������֪��E��ȥ1����HBr������ȥ��Ӧ����F����E�����к���1��Brԭ�ӣ�E����ˮ�ⷴӦ����D��DΪ������ֻ��һ����ԭ�ӣ���DΪһԪ����C������ԭ��Ӧ����D��C��D��E��F������̼ͬԭ����Ŀ����������5��̼ԭ�ӣ�AΪ���࣬ˮ������C��B����֪CΪ���ᣬ����DΪһԪ������CΪһԪ���ᣬMr��C��=102��C�ķ����к���4����ԭ�ӣ�����CΪ��CH3��2CHCH2COOH����DΪ��CH3��2CHCH2CH2OH��EΪ��CH3��2CHCH2CH2Br��FΪ��CH3��2CHCH=CH2����AΪ ���ݴ˽��
���ݴ˽��
| 70 |
| 12 |
 ���ݴ˽��
���ݴ˽�����
�⣺FΪ������Է�������Ϊ70���������Cԭ�������ĿΪ
=5��10����F�ķ���ʽ��C5H10����D��E��F��ת����֪��EΪ����������E��F����Է���������֪��E��ȥ1����HBr������ȥ��Ӧ����F����E�����к���1��Brԭ�ӣ�E����ˮ�ⷴӦ����D��DΪ������ֻ��һ����ԭ�ӣ���DΪһԪ����C������ԭ��Ӧ����D��C��D��E��F������̼ͬԭ����Ŀ����������5��̼ԭ�ӣ�AΪ���࣬ˮ������C��B����֪CΪ���ᣬ����DΪһԪ������CΪһԪ���ᣬMr��C��=102��C�ķ����к���4����ԭ�ӣ�����CΪ��CH3��2CHCH2COOH����DΪ��CH3��2CHCH2CH2OH��EΪ��CH3��2CHCH2CH2Br��FΪ��CH3��2CHCH=CH2����AΪ ��
��
��1����B�Ľṹ��ʽ��֪��B�ķ���ʽΪC10H18O��F�ķ���ʽΪC5H10��
�ʴ�Ϊ��C10H18O��C5H10��
��2����������������C��ͬ���칹��Ľṹ��ʽ������������ ���ܷ���������Ӧ��Ϊ���ᶡ�������ܵĽṹ�У� ��HCOOCH2CH��CH3��2��HCOOCH��CH3��CH2CH3��HCOOC��CH3��3���ʴ�Ϊ��
��HCOOCH2CH��CH3��2��HCOOCH��CH3��CH2CH3��HCOOC��CH3��3���ʴ�Ϊ�� ��HCOOCH2CH��CH3��2�ȣ�
��HCOOCH2CH��CH3��2�ȣ�
��3��D��E�Ǽ��������£���������������Ƶ�ˮ��Һ����ȡ����Ӧ���ɴ����廯�ƣ���Ӧ��ѧ����ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
| 70 |
| 12 |
 ��
����1����B�Ľṹ��ʽ��֪��B�ķ���ʽΪC10H18O��F�ķ���ʽΪC5H10��
�ʴ�Ϊ��C10H18O��C5H10��
��2����������������C��ͬ���칹��Ľṹ��ʽ������������ ���ܷ���������Ӧ��Ϊ���ᶡ�������ܵĽṹ�У�
 ��HCOOCH2CH��CH3��2��HCOOCH��CH3��CH2CH3��HCOOC��CH3��3���ʴ�Ϊ��
��HCOOCH2CH��CH3��2��HCOOCH��CH3��CH2CH3��HCOOC��CH3��3���ʴ�Ϊ�� ��HCOOCH2CH��CH3��2�ȣ�
��HCOOCH2CH��CH3��2�ȣ���3��D��E�Ǽ��������£���������������Ƶ�ˮ��Һ����ȡ����Ӧ���ɴ����廯�ƣ���Ӧ��ѧ����ʽΪ��
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���������⿼�����л�����ƶϣ��ؼ�ȷ��F�ķ���ʽ�����ݷ�Ӧ�������������Ʒ������Ʒ����ϵķ��������ƶϣ���Ҫѧ���������չ����ŵ�������ת�����Ѷ��еȣ�

��ϰ��ϵ�д�
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д� ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
�����Ŀ
�����ƶ���ȷ���ǣ�������
| A��Na2O��Na2O2���Ԫ����ͬ���� CO2��Ӧ����Ҳ��ͬ |
| B��������ˮ�����ԣ������еμ�������ɫʯ����Һ���������Һ�ʺ�ɫ |
| C��CO��NO��NO2���Ǵ�����Ⱦ���壬�ڿ����ж����ȶ����� |
| D��SiO2 ���������������NaOH��Һ��Ӧ |
ijͬѧ��Na2CO3����0.10mol/L Na2CO3��Һ�Ĺ�����ͼ��ʾ������Ϊ��ͬѧ�Ĵ����У�������


| A���٢ݢ� | B���ڢܢ� |
| C���٢� | D���ݢޢ� |