��Ŀ����
13�� ҽ�����̷���FeSO4•7H2O��������ȱ����ƶѪ����Чҩ��ij��ѧ��ȤС����̷����������µ�̽����
ҽ�����̷���FeSO4•7H2O��������ȱ����ƶѪ����Чҩ��ij��ѧ��ȤС����̷����������µ�̽�������Ʊ���Ʒ��
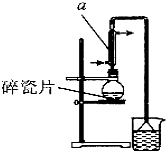
��С���ɷ���м������������ͭ�������������ʣ�������ͼ��ʾװ���Ʊ� FeSO4•7H2O���壬�������£�
��1��Ԥ�������Ƚ�����м���뵽���� Na2CO3��Һ��ϴ�ӣ�Ŀ����ϴȥ��м��������ۣ�Ȼ����м��ˮϴ��2��3�飮
��2����ϴ�Ӻ�ķ���м���뵽Բ����ƿ�У�������ͨ��N2
��3���ټ�������ϡ���ᣬ�����¶�50�桫80��֮�䣬��ַ�Ӧ��Բ����ƿ��ʣ��Ĺ���ΪCu��ʵ���ҳ������軯����Һ���鷴Ӧ���ɵ�Fe2+��д���÷�Ӧ�����ӷ�Ӧ����ʽ��3Fe2++2[Fe��CN��6]�TFe3[Fe��CN��6]2����
��4����ȡ��Ʒ�������裨3���з�Ӧ��Ļ�����м�����������ˮ�����ȹ��ˣ���ȴ�ᾧ���˳����壬��������ˮϴ��2��3�Σ�������ֽ���������ɣ��ܱձ��森
���ⶨFeSO4•7H2O������
��1����ȡ������Ʒ10.0g������������ϡ�����У����100mL��Һ��ȷ��ȡ25.00mL��Һ������ƿ�У���0.1000mol/L KMnO4����Һ�ζ��ζ�3�Σ�ƽ������10.00mL��Һ������Ʒ��FeSO4•7H2O����������Ϊ55.6%������֪ Mr��FeSO4•7H2O��=278����
��2�����������ƫС����������ڶ���ʱ���ӣ�����ӡ������ӡ���������
���� I������ͭ�������������ᷴӦ��������ͭ����������������������ͭ����������Ӧ��������������ͭ���ʣ�
��1��̼���Ƶ�ˮ��Һ�Լ��ԣ����Գ�ȥ��м��������ۣ�
��3������ͭ�������������ᷴӦ��������ͭ����������������������ͭ����������Ӧ��������������ͭ���ʣ����軯�������������������������軯����������
��1��Fe2+��Fe3+����Ԫ�ػ��ϼ�����1�ۣ�MnO4-��Mn2+����Ԫ�ؽ���5�ۣ����ϼ�������С������Ϊ5�����ݹ�ϵʽ5Fe2+��MnO4-������25mL��Һ����Fe2+�����ʵ���������������Ʒ��FeSO4•7H2O���������ٸ�����������������㣻
��2������ʱ���Ӷ�ȡ����Һ���ƫ��
��� �⣺I����1��̼���Ƶ�ˮ��Һ�Լ��ԣ����Գ�ȥ��м��������ۣ�
�ʴ�Ϊ��ϴȥ��м��������ۣ�
��3������ͭ�������������ᷴӦ��������ͭ����������������������ͭ����������Ӧ��������������ͭ���ʣ����軯�������������������������軯�������������ӷ���ʽ��3Fe2++2[Fe��CN��6]�TFe3[Fe��CN��6]2����
�ʴ�Ϊ��Cu��3Fe2++2[Fe��CN��6]�TFe3[Fe��CN��6]2����
��1���ⶨ��Ʒ��Fe2+�ĺ���������������������KMnO4��Һ�ζ���Fe2+��Fe3+����Ԫ�ػ��ϼ�����1�ۣ�MnO4-��Mn2+����Ԫ�ؽ���5�ۣ����ϼ�������С������Ϊ5����Fe2+ϵ��Ϊ5��MnO4- ϵ��Ϊ1������Ԫ���غ��֪Mn2+��Fe3+ϵ���ֱ�Ϊ1��5�����ݵ���غ��֪ȱ��ΪH+��H+��ϵ��Ϊ2+3��5-[2��5-1]=8������HԪ���غ��֪H2Oϵ����4�����Է�Ӧ���ӷ���ʽΪ5Fe2++MnO4-+8H+=1Mn2++5Fe3++4H2O��
��Fe2+�����ʵ���Ϊxmol����
5Fe2+������������MnO4-��
5 1
xmol 0.01L��0.1mol/L
����x=$\frac{0.01��0.1mol/L��5}{1}$=0.005mol��
������Ʒ��FeSO4•7H2O������0.005mol��4��278g/mol=5.56g������4g��Ʒ��FeSO4•7H2O����������Ϊ$\frac{5.56g}{10g}$��100%=55.6%��
�ʴ�Ϊ��55.6%��
��4������ʱ�������ƫ��Ũ��ƫС��
�ʴ�Ϊ�����ӣ�
���� ���⿼�������ӷ���ʽ����д����ȷ���ʵ����ʼ�������Ӧ��ʵ���ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�| A�� | �ڱ�״���£�22.4Lˮ������ԼΪ18g | |
| B�� | 22.4LN2��6.02��1023���������� | |
| C�� | 2gH2ռ�е����ԼΪ22.4L | |
| D�� | 22g������̼���״����11.2LHCl���庬����ͬ�ķ����� |
| A�� | CH3COOH��aq��+NaOH��aq���TCH3COONa��aq��+H2O��l����H=-57.3kJ/mol | |
| B�� | KOH��aq��+$\frac{1}{2}$H2SO4��aq��=$\frac{1}{2}$K2SO4��aq��+H2O��l����H=+57.3kJ/mol | |
| C�� | C8H18��l��+$\frac{25}{2}$ O2 ��g��=8CO2 ��g��+9H2O��g����H=-5518kJ/mol | |
| D�� | 2C8H18��g��+25O2 ��g��=16CO2 ��g��+18H2O��1����H=-11036kJ/mol |
 ������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ� 1-�嶡��ķ�Ӧ��ʵ��װ�����£�
������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ� 1-�嶡��ķ�Ӧ��ʵ��װ�����£�NaBr+H2SO4$\frac{\underline{\;��\;}}{\;}$HBr+NaHSO4��
C4H9-OH+HBr$\stackrel{��}{��}$C4H9-Br+H2O��
���ܴ��ڵĸ���Ӧ�У�����Ũ����Ĵ�������ˮ����ϩ���ѣ�Br-��Ũ��������ΪBr2�ȣ�
�й������б����£�
| �۵�/�� | �е�/�� | �ܶ�/g•cm-3 | |
| ������ | -89.53 | 117.25 | 0.81 |
| 1-�嶡�� | -112.4 | 101.6 | 1.28 |
��2���Ʊ������У������Ũ�����������ʵ�ϡ�ͣ���Ŀ����abc������ĸ��ţ���
a�����ٸ�����ϩ���ѵ�����
b������ Br2������
c������ HBr �Ļӷ�
d��ˮ�Ƿ�Ӧ�Ĵ���
��3����Ӧ��������Ӧ�������1-�嶡����������Ӧ��ȡ��������õ��ϴ���1-�嶡�飬����װ�ó����õ������ܡ��¶ȼơ�ţ�ǹܡ���ƿ������Ҫ�IJ��������Ǿƾ��ơ�������ƿ��
��4������Ӧ������õ��Ļ���ᆳ����������õ��ϴ���1-�嶡��IJ����У����ܺ��е�������Ҫ��ˮ��
��5����1-�嶡���Ʒ���ڷ�Һ©���м�ˮ�����ã��������²㣨��ϲ㡱�����²㡱���ֲ㡱����
��6��ijʵ��С����ȡ1-�嶡��ʱ����Բ����ƿ�м���7.4g��������13.0g NaBr��������Ũ���ᣬ�����Ƶ�1-�嶡��9.6g����1-�嶡��IJ�����70%������2 λ��Ч���֣���
��1����֪��N2��g��+O2��g��=2NO��g����H=+180.5kJ•mol-1
2C��s��+O2��g��=2CO��g����H=-221.0kJ•mol-1
C��s��+O2��g��=CO2��g����H=-393.5kJ•mol-1
��β��ת���ķ�Ӧ֮һ��2NO��g��+2CO��g��=N2��g��+2CO2��g����H=-746.5 kJ•mol-1��
����֪��N2��O2�����л�ѧ���ļ��ֱܷ���946kJ•mol-1��497kJ•mol-1����NO�����л�ѧ���ļ���Ϊ631.25kJ•mol-1��
��2��ij�о���ѧϰС���ڼ�����Ա��ָ���£���ij�¶�ʱ������������̽��ij�ִ��������µķ�Ӧ���ʣ������崫������ò�ͬʱ���NO��COŨ�������

| ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
| c��NO������10-4mol•L-1�� | 10.0 | 4.50 | 2.50 | 1.50 | 1.00 | 1.00 |
| C��CO������10-3mol•L-1�� | 3.60 | 3.05 | 2.85 | 2.75 | 2.70 | 2.70 |
��ǰ3s�ڵ�ƽ����Ӧ����v ��N2��=1.42��10-4mol•L-1•s-1��
���ڸ��¶��£���Ӧ��ƽ�ⳣ��K=5000����ֻд����������
�۸ÿ��淴Ӧ��S��0�����������������=�������ڵ��£�����¡��������¡����κ��¶ȡ��������Է����У�
��3��CO��������ȼ�ϵ��Ϊ����ԭ������װ����ͼ��ʾ���õ���е����Ϊ������-�����ƣ�����O2-�����ڹ������NASICON�������ƶ�������˵���������B

A�������ĵ缫��ӦʽΪ��CO+O2--2e-=CO2
B������ʱ�缫b��������O2-�ɵ缫a����缫b
C������ʱ�����ɵ缫aͨ������������缫b
D����������ͨ���ĵ���Խ��β����CO�ĺ���Խ�ߣ�
C6H12O6+6O2+38H3PO4+38ADP��6CO2+44H2O+38ATP
ATP+H2O��ADP+H3PO4����H=-30.6kJ/mol
�ɴ˿�֪����������������ȫ�����ֽ�ʱ��������������Ϊ��������
| A�� | 40.5% | B�� | 60.6% | C�� | 81.0% | D�� | 100% |
| A�� | 5min����O2��ʾ�ķ�Ӧ����Ϊ0.12mol/��L•min�� | |
| B�� | �����������ʹ�÷�Ӧ�Ļ�ѧ��Ӧ���ʼӿ죬��H��С | |
| C�� | SO2��ƽ��Ũ��Ϊ0.12mol/L | |
| D�� | �ﵽƽ��ʱ���������������������÷�Ӧ�Ļ�ѧ��Ӧ���ʼ��� |
 Ϊʵ�֡����ܼ��š��͡���̼���á���һ���������ν�CO2ת��Ϊ��������Դ��Ŀǰ����ҵ�ϳ���CO2������ȼ�ϼ״����ֽ�������ʵ�飺�����Ϊl L���ܱպ��������У�����l mol CO2��3mol H2��һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ/mol��
Ϊʵ�֡����ܼ��š��͡���̼���á���һ���������ν�CO2ת��Ϊ��������Դ��Ŀǰ����ҵ�ϳ���CO2������ȼ�ϼ״����ֽ�������ʵ�飺�����Ϊl L���ܱպ��������У�����l mol CO2��3mol H2��һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ/mol�� CuCl��Ӧ�ù㷺���л��ϳɴ������ɲ�ȡ��ͬ������ȡ��
CuCl��Ӧ�ù㷺���л��ϳɴ������ɲ�ȡ��ͬ������ȡ��