��Ŀ����
20�������ѣ�CH3OCH3������Ϊ21���͵���ࡢ��Ч��Դ��I����1���ϳɶ����ѷ�Ӧһ��3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g����H=-247KJ/mol
һ�������¸÷�Ӧ���ܱ������дﵽƽ���Ҫ�ӿ췴Ӧ������Ҫ���H2��ת���ʣ����Բ�ȡ�Ĵ�ʩ��D��
A������ B���Ӵ��� C������������N2
D������COŨ�� E�������������
��2�����������������䣬ֻ�ı�����������ʹH2����������������C��
A��ʹ�ø�Ч���� B������ѹǿ
C�������¶� D�����������ٳ���1mol ��CH3OCH3����1molCO2
��3����һ���¶��£������жϸ÷ֽⷴӦ�Ѿ��ﵽ��ѧƽ�����ABD��
A��V����CO���sV����CO2��=3�s1B�������ܱ���������ѹǿ����
C�������ܱ������л��������ܶȲ���D���ܱ������������������������
��4���ϳɶ����ѷ�Ӧ����2CH3OH��g��?CH3OCH3��g��+H2O��g�����ڲ�ͬ�¶��£��ֱ���1L�ܱ������м��벻ͬ���ʵ�����CH3OH����Ӧ�ﵽƽ�⣬��ø���ֵ�Ũ���������
| ʵ���� | �¶�/K | ƽ��Ũ��mol/L | ||
| CH3OH | CH3OCH3 | H2O | ||
| l | 403 | 0.01 | 0.2 | 0.2 |
| 2 | 453 | 0.02 | 0.3 | 0.4 |
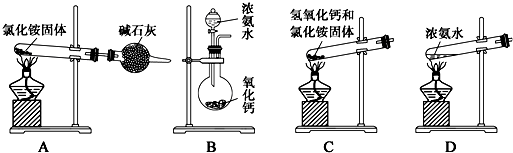
������ȼ�ϵ�صĹ���ԭ����ͼһ��ʾ��

��1���õ�ظ����ĵ缫��ӦʽΪCH3OCH3+3H2O-12e-=12H++2CO2��
��2�����������Ϊ��Դ��ͨ��������ͼ������������X��YΪʯī��aΪ1L0.1mol/LKCl��Һ������������0.224L����״���£�����ʱ����Һ��pHΪ12����������Һ������仯��
��3������ʱ����������2�����һ��ʱ���ȡ25mL����������Һ���μ�0.2mol/L ����õ�ͼ����������������ʧ����������ˮ����Һ����仯���Բ��ƣ���
����ͼ����B��pH=7����ζ��յ���AB���䣨�AB������BC����CD������
��C����Һ�и�����Ũ�ȴ�С��ϵ��c��CH3COO-����c��K+����c��H+����c��OH-����
���� ��1��Ҫ�ӿ췴Ӧ���ʣ�Ӧ���¡���ѹ������Ũ�Ȼ���������Ҫ���H2��ת���ʣ�Ӧʹƽ��������Ӧ�����ƶ���
��2���÷�Ӧ���������ʵ�����С�ķ��ȷ�Ӧ��ҪʹH2�������������Ӧʹƽ�������ƶ���
��3����Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȣ������Ũ�Ȳ��䣬�ݴ˷�����
��4����ƽ�ⳣ���ļ��㹫ʽ���㼴�ɣ�
��1������������ԭ��Ӧ�������õ��ӱ���ԭ�����������ƶ������֪���缫Ϊ�������Ҳ�缫Ϊ������
��2�����KCl��Һ������KOH��������������
��3������Ϊ���ᣬǡ���к�ʱ��Һ�ʼ��ԣ�pH��7��C������������Һ�����ԣ�
��� �⣺��1��A������Ӧ���ȣ������¶ȿ�ʹƽ�����������ƶ�������Ӧ���ʼ�������A����
B���������ƽ�ⲻ�ƶ�����B����
C������������N2��ƽ�ⲻ�ƶ�����C����
D������COŨ�ȣ�������ת�������ߣ��ҷ�Ӧ���ʼӿ죬��D��ȷ��
E������������ѣ���ʹƽ�����������ƶ�������Ӧ���ʼ�������E����
�ʴ�Ϊ��D��
��2��A��������Ӱ��ƽ���ƶ���ʹ�ø�Ч����ֻ�Ǵﵽƽ���ʱ�����̣���A����
B����ѹ���������ѹǿ��ƽ�������ƶ�������������������٣���B����
C�������¶�ƽ�������ƶ��������������������C��ȷ��
D�����������ٳ���1mol ��CH3OCH3����1molCO2���൱�ڼ�ѹ�����������������С����D����
�ʴ�Ϊ��C��
��3��A��V����CO���sV����CO2��=3�s1��˵�����淴Ӧ������ȣ���Ӧ�ﵽƽ��״̬����A��ȷ��
B�������ܱ���������ѹǿ���䣬˵�������Ũ�Ȳ��䣬��Ӧ�ﵽƽ��״̬����B��ȷ��
C������������䣬�����������䣬�ܶ�ʼ�ղ��䣬���Ժ����ܱ������л��������ܶȲ��䲻��˵����Ӧ�ﵽƽ��״̬����C����
D���ܱ�����������������������䣬˵�������Ũ�Ȳ��䣬��D��ȷ��
�ʴ�Ϊ��ABD��
��4��ƽ��ʱK��403��=$\frac{0.2��0.2}{��0.01��^{2}}$=400���ʴ�Ϊ��400��
��1������������ԭ��Ӧ�������õ��ӱ���ԭ���缫��ӦΪ4H++O2+4e-=2H2O�����������ƶ������֪���缫Ϊ�������Ҳ�缫Ϊ������������ӦΪ
CH3OCH3+3H2O-12e-=12H++2CO2������H+��pH����
�ʴ�Ϊ��CH3OCH3+3H2O-12e-=12H++2CO2��
��2�����KCl��Һ������KOH���������������ܷ�ӦʽΪ2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$Cl2��+H2��+2OH-������������0.224L����״���£�����ʱ������KOH0.01mol��������pHΪ12���ʴ�Ϊ��12��
��3���ٴ���Ϊ���ᣬǡ���к�ʱ��Һ�ʼ��ԣ�pH��7��ӦΪ��AB֮�䣬�ʴ�Ϊ��AB��
��C������������Һ�����ԣ�Ӧ��c��CH3COO-����c��K+����c��H+����c��OH-�����ʴ�Ϊ��c��CH3COO-����c��K+����c��H+����c��OH-����
���� ���⿼���Ϊ�ۺϣ��漰��ѧƽ����ƶ���ԭ���������Լ�����Ũ�ȵĴ�С�Ƚϣ���Ŀ�Ѷ��еȣ�ע����յ缫����ʽ����д�Լ�ƽ�ⳣ�������壮

| A�� | ����������Һ��ϡ H2SO4 ��Ӧ��Ba2++SO42-+H++OH-�TBaSO4��+H2O | |
| B�� | ϡ H2SO4�����۷�Ӧ��2Fe+6H+�T2Fe3++3H2�� | |
| C�� | ��������Һ�����۷�Ӧ��2Ag++Fe�TFe2++2Ag | |
| D�� | ̼��������ᷴӦ��CO32-+2H+�TH2O+CO2�� |
| A�� | S��SO3��H2SO4 | B�� | Fe��FeCl3��FeCl2 | ||
| C�� | Si��H2SiO3��Na2SiO3 | D�� | Al2O3��Al��OH��3��NaAlO2 |

| A�� | ��ӦI2��aq��+I-��aq��?I3-��aq����H��0 | |
| B�� | �¶�ΪT1ʱ�����ƽ����ϵ�м���KI���壬ƽ�ⲻ�ƶ� | |
| C�� | ��T1ʱ����Ӧ���е�״̬dʱ��һ����v����v�� | |
| D�� | ״̬a��״̬b��ȣ�״̬bʱI2��ת���ʸ��� |
| A�� | �Ʊ���Ĺ����漰��������Ӧ | |
| B�� | ���͡����͡�ֲ���Ͷ���̼�⻯���� | |
| C�� | ��5��̼ԭ�ӵ��л�������������γ�4��̼̼���� | |
| D�� | �����ʵ�ˮ�����֬�����������ɸ߷�������С���ӵĹ��� |
| A�� | NH4+��NO3-��CO32-��Na+ | B�� | Na+��A13+��H+��Cl- | ||
| C�� | NO3-��NH4+��K+��Cl- | D�� | NO3-��K+��AlO2-��OH- |