��Ŀ����
14��ijˮ��Һ�к������������е������֣�K+��Cl-��Ca2+��Zn2+��CO32-��SO42-����ȡ������Һ��100mL�ֱ��������ʵ�飺�ٵ�һ�ݼ���AgNO3��Һ�г�������
�ڵڶ��ݼ�����BaCl2��Һ�ó�����6.63g������������ϴ�ӡ������������Ϊ4.66g����������Һ�м���AgNO3��Һ�г�������
��������ʵ�飬�����й�ԭ��Һ���۶ϲ���ȷ���ǣ�������
| A�� | Cl-һ������ | |
| B�� | CO32-��SO42-һ�����ڣ��Ҷ��ߵĸ�����Ϊ1��2 | |
| C�� | Zn2+��Ca2+һ�������� | |
| D�� | 100mL��Һ��K+��������С��2.34g |
���� �ٵ�һ�ݼ���AgNO3��Һ�г�����������ɫ��������Ϊ̼���������������Ȼ�����˵����Һ�п��ܴ���Cl-��CO32-��SO42-��
�ڵڶ��ݼ�����BaCl2��Һ�ó�����6.63g������������ϴ�ӡ������������Ϊ4.66g��˵�����ɵij����ܲ����������ᣬ������Һ�к���CO32-��SO42-����Zn2+��Ca2+��CO32-�����棬����û��Zn2+��Ca2+���ɵ���غ��֪��һ����������ΪK+��������Һ�м���AgNO3��Һ�г�������������ΪAgCl�������Ȼ������������ӣ����Ͽ�֪��Һ��һ����CO32-��SO42-��������Cl-��һ��û��Zn2+��Ca2+���Դ������
��� �⣺A������ȷ���Ƿ�Cl-����A����
B��һ����CO32-��SO42-���Ҷ��ߵĸ�����Ϊ$\frac{6.63-4.66g}{197g/mol}$��$\frac{4.66g}{233g/mol}$=1��2����B��ȷ��
C�������ӹ����֪Zn2+��Ca2+һ�������ڣ���C��ȷ��
D��CO32-��SO42-�����ʵ����ֱ�Ϊ0.01mol��0.02mol���ɵ���غ��֪��������������Ϊ0.01mol��2+0.02mol��2=0.06mol����100mL��Һ��K+��������С��0.06mol��39g/mol=2.34g����D��ȷ��
��ѡA��
���� ���⿼��������ƶϣ�Ϊ��Ƶ���㣬���շ����ķ�Ӧ�����ӹ��桢Ԫ�ػ�����֪ʶΪ���Ĺؼ������ط������ƶ������Ŀ��飬ע��Ԫ�ػ�����֪ʶ���ۺ�Ӧ�ã���Ŀ�ѶȲ���


| A�� | пƬ����������Ӧ������ | |
| B�� | ���Ӵ���Ƭ������������пƬ | |
| C�� | ��Ƭ�ϵ缫��ӦΪ��O2+2H2O+4e��4OH- | |
| D�� | ��װ�ÿ������о���ӵ������������� |
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2 054 | 1 535 | 1 462 |
| �е�/�� | 2 467 | 2 980 | 2 750 | - |
��2�����һ����ʵ�鷽����֤���������õĿ�״�������к��н���������ʵ�������Լ���NaOH��Һ����Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
��3��ʵ�����ܽ��������������Լ��������˵��Լ���B������ţ���
A��Ũ���� B��ϡ���ᡡ������C��ϡ���� D������������Һ
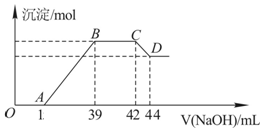
��ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�ͣ�ijͬѧȡһ������������������һ������ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���6mol•L-1������������Һ����������������Һ�������mL��������ij��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ���Իش��������⣺
��1�������⣬��д���������������ϡ�����ᷴӦ�����ӷ���ʽ��8Fe+30H++3NO3-�T8 Fe3++3NH4++9 H2O
��2��ͼ��OA��û�г������ɣ��˽η�����Ӧ�����ӷ���ʽΪH++OH-�TH2O��
��3����BC�Σ����������ʵ���û�б仯����˽η�����Ӧ�����ӷ���ʽΪNH4++OH-�TNH3•H2O��
��4������������Ԫ�ص����ʵ���Ϊ0.012mol��
��5��B���Ӧ�ij��������ʵ���Ϊ0.048mol��A���Ӧ������������Һ�����Ϊ15mL��
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | O |
| 2 | �� | �� | �� | �� | ||||
| 3 | �� | �� | �� | �� | �� | �� | ⑪ | ⑫ |
��2������ЩԪ���У�Na����Ԫ�ط�����д����ͬ��������õĽ���Ԫ�أ�F������õķ�
����Ԫ�أ�
��3����ЩԪ�ص����������Ķ�Ӧˮ������HClO4������ǿ��NaOH������ǿ�����γ��������������Ԫ����Al��
��4���ȽϢ���Ļ�ѧ���ʣ�Na�����ã�
| A�� | �����������������ۻ� | B�� | �����¶�����ͭ��Ӧ�������� | ||
| C�� | ¶���ڿ����У���Һ���������� | D�� | ¶���ڿ����У���ҺŨ�Ⱦ����� |
 ijѧϰС��ͬѧ������ͼװ������֤ͬ����Ԫ�طǽ����Եı仯���ɣ�
ijѧϰС��ͬѧ������ͼװ������֤ͬ����Ԫ�طǽ����Եı仯���ɣ�


