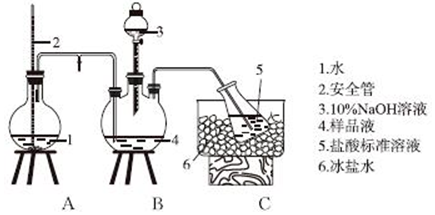
��Ŀ����
2�����ȷ�Ӧ������һ����Ҫ���ʣ���������;ʮ�ֹ㷺�������ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â����ֽ©�����²����մ���������������ɳ�С�����֪��Al��Al2O3��Fe��Fe2O3���۵㡢�е����������| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2 054 | 1 535 | 1 462 |
| �е�/�� | 2 467 | 2 980 | 2 750 | - |
��2�����һ����ʵ�鷽����֤���������õĿ�״�������к��н���������ʵ�������Լ���NaOH��Һ����Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
��3��ʵ�����ܽ��������������Լ��������˵��Լ���B������ţ���
A��Ũ���� B��ϡ���ᡡ������C��ϡ���� D������������Һ
��ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�ͣ�ijͬѧȡһ������������������һ������ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���6mol•L-1������������Һ����������������Һ�������mL��������ij��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ���Իش��������⣺
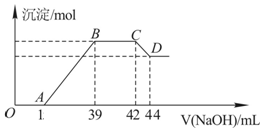
��1�������⣬��д���������������ϡ�����ᷴӦ�����ӷ���ʽ��8Fe+30H++3NO3-�T8 Fe3++3NH4++9 H2O
��2��ͼ��OA��û�г������ɣ��˽η�����Ӧ�����ӷ���ʽΪH++OH-�TH2O��
��3����BC�Σ����������ʵ���û�б仯����˽η�����Ӧ�����ӷ���ʽΪNH4++OH-�TNH3•H2O��
��4������������Ԫ�ص����ʵ���Ϊ0.012mol��
��5��B���Ӧ�ij��������ʵ���Ϊ0.048mol��A���Ӧ������������Һ�����Ϊ15mL��
���� I����1�������۵�����ͣ���������Һ̬��һ�������ʢ��ɳ�ӵ��������γɺϽ�
��2����������NaOH��Һ��Ӧ���������ʵ�鷽����
��3��A��Ũ������ʹ�������ۻ�������ʹ�Ͻ��ܽ⣻
B�����ý�����ϡ���ᷴӦ��
C��ϡ�����������Ӧ������Ⱦ�����壻
D��������NaOH��Һ��Ӧ��
��1����������ϡ���ᷴӦ������������������ƶϳ���ԭ����ӦΪNH4+��
��2��OA ֮��û�г������ɣ�˵�����������OA ֮�䷢���ķ�Ӧ����кͷ�Ӧ���Դ���д��Ӧ�����ӷ���ʽ��
��3��BC�γ���������û�з����仯��ΪNH4NO3��NaOH��Ӧ���Դ���д���ӷ���ʽ��
��4��CD�η����ķ�Ӧ��Al��OH��3 +OH-=AlO2-+2H2O���������ĵ�NaOH�����ʵ����ɵ�Al��OH��3�ģ���Ϊ����������Ԫ�ص����ʵ�����
��5��B���Ӧ�ij���ΪFe��OH��3��Al��OH��3����Fe��Al��M���棬���й�ϵʽ8M��3NH4+��3OH-��ͨ��BC�����ĵ�OH-����������������ʵ�����ͨ��Fe3++3OH-=Fe��OH��3����Al3++3OH-=Al��OH��3������AB�����ĵ�����������Һ����������ɵõ�A���Ӧ������������Һ�������
��� �⣺����1�������۵�����ͣ���������Һ̬��һ�������ʢ��ɳ�ӵ��������γɺϽ��������ȷ�Ӧ���õ���������Ӧ�������Ͻ�
�ʴ�Ϊ��������
��2������NaOH��Һ��Ӧ�������壬��Ӧ�Ļ�ѧ����ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2�������Կ���NaOH��Һ�������õĿ�״�������к��н�������
�ʴ�Ϊ��NaOH��Һ��2Al+2OH-+2H2O=2AlO2-+3H2����
��3��A��Ũ������ʹ�������ۻ�������ʹ�Ͻ��ܽ⣬��A����
B�����ý�����ϡ���ᷴӦ����������ܽ���ϡ�����У���B��ȷ��
C��ϡ�����������Ӧ������Ⱦ�����壬�������ѡ��C����
D��������NaOH��Һ��Ӧ�����ܽ�������ȫ���ܽ⣬��D����
�ʴ�Ϊ��B��
��1����������ϡ���ᷴӦ������������������ƶϳ���ԭ����ӦΪNH4+�����������ϡ�����ᷴӦ�����ӷ���ʽΪ8Fe+30H++3NO3-�T8 Fe3++3NH4++9 H2O��
�ʴ�Ϊ��8Fe+30H++3NO3-�T8 Fe3++3NH4++9 H2O��
��2��OA ֮��û�г������ɣ�˵�����������OA ֮�䷢������������������Ƶ��кͷ�Ӧ�����ӷ���ʽΪH++OH-�TH2O��
�ʴ�Ϊ��H++OH-�TH2O��
��3��BC�γ���������û�з����仯��ΪNH4NO3��NaOH��Ӧ�����ӷ���ʽΪNH4++OH-�TNH3•H2O��
�ʴ�Ϊ��NH4++OH-�TNH3•H2O��
��4��CD�η����ķ�Ӧ��Al��OH��3 +OH-=AlO2-+2H2O��n��NaOH��=0.002L��6mol/L=0.012mol����n��Al��OH��3��=0.012mol��������������Ԫ�ص����ʵ���Ϊ0.012mol��
�ʴ�Ϊ��0.012��
��5����8Fe+30H++3NO3-�T8 Fe3++3NH4++9 H2O��8Al+30H++3NO3-�T8 Al3++3NH4++9 H2O��NH4++OH-�TNH3•H2O�ɵù�ϵʽ8��Fe��Al����3NH4+��3OH-��BC�����ĵ�OH-�����ʵ���Ϊ6mol•L-1����42-39����10-3L=0.018mol����Fe��Al�����ʵ���֮��Ϊ0.018mol��$\frac{8}{3}$=0.048mol������B���Ӧ�ij���Fe��OH��3��Al��OH��3�����ʵ���Ϊ0.048mol��AB�����ĵ�����������Һ���ʵ���Ϊ0.048mol��3=0.144mol��AB�����ĵ��������Ƶ����Ϊ$\frac{0.144mol}{6mol/L}$=0.024L=24ml������A���Ӧ������������Һ�����Ϊ39-24=15��
�ʴ�Ϊ��0.048��15��
���� ���⿼���Ϊ�ۺϣ��漰���ȷ�Ӧ������������ķ�Ӧ�����ӷ���ʽ����д��������ԭ��Ӧ����ѧ�����֪ʶ�㣬��Ŀ�Ѷ��еȣ�������ؼ����жϳ�����Ļ�ԭ�����Լ�����ת���غ�����ã�

 �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�| A�� | ��⾫��ͭ�������·ͨ��NA������ʱ��������������32 g | |
| B�� | ��Ӧ3H2��g��+N2��g��?2NH3��g����H=-92kJ•mol-1�����ų����� 9.2 kJʱ��ת�Ƶ���0.6 NA | |
| C�� | lmol•L1���Ȼ�ͭ��Һ�У���Cl-����ĿΪ2Na����Cu2+����ĿΪNA | |
| D�� | ��״���£�NO��O2��11.2L��ϳ�ַ�Ӧ����������ķ�������Ϊ0.75 NA |
| A�� | ��״���£�3.36LC2H4��C3H6�Ļ�������к���̼̼˫������ĿΪ0.15NA | |
| B�� | 0.1mol•L-1��NH4��2SO4��Һ��0.2mol•L-1NH4Cl��Һ�е�NH4+��Ŀ��ͬ | |
| C�� | H2��CO�������8.96L������O2�г��ȼ������O2������Ϊ0.2NA | |
| D�� | ��0.1molNH4HSO4����Һ�У���������Ŀ�Դ���0.21NA |
�ֱ�ȡ���ǵ�ˮ��Һ����ʵ�飬������£�
| ������ | Na+ | Al3+ | Fe3+ | Cu2+ | Ba2+ |
| ������ | OH- | Cl- | CO32- | NO3- | SO42- |
��B ��Һ�� E ��Һ��Ϻ�������ɫ������ͬʱ�����������壻
������ C ��Һ�� D ��Һ��Ϻ������ɫ���������� C ��Һ�� D ��Һ��Ϻ�������
��B ��Һ�� D ��Һ��Ϻ�������
�ݽ� 38.4g Cu ƬͶ��װ������ D ��Һ���Թ��У�Cu Ƭ���ܽ⣬�ٵμ� 1.6mol/L ϡ H2SO4��Cu ���ܽ⣬�ܿڸ����к���ɫ������֣�
��1���ݴ��ƶ� A �Ļ�ѧʽΪ��ACuSO4��BFeCl3
��2��д������ C �� D ������Ӧ�����ӷ���ʽAl3++4OH-=AlO2-+2H2O��
��3��B ��Һ�е���ʯ����Һ����������Һ��죬ԭ����Fe3++3H2O?Fe��OH��3+3H+�������ӷ���ʽ˵����
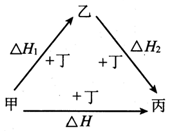 �ס��ҡ�����������������һ �������µ�ת����ϵ��ͼ��ʾ��������Ϊ��Ӧ����������ʵ������ʱ����H=��H1+��H2����ס��������ǣ�������
�ס��ҡ�����������������һ �������µ�ת����ϵ��ͼ��ʾ��������Ϊ��Ӧ����������ʵ������ʱ����H=��H1+��H2����ס��������ǣ�������| A�� | S��SO3 | B�� | AlCl3��NaAlO2 | C�� | Na��Na2O2 | D�� | NaOH��Na2CO3 |
�ٵ�һ�ݼ���AgNO3��Һ�г�������
�ڵڶ��ݼ�����BaCl2��Һ�ó�����6.63g������������ϴ�ӡ������������Ϊ4.66g����������Һ�м���AgNO3��Һ�г�������
��������ʵ�飬�����й�ԭ��Һ���۶ϲ���ȷ���ǣ�������
| A�� | Cl-һ������ | |
| B�� | CO32-��SO42-һ�����ڣ��Ҷ��ߵĸ�����Ϊ1��2 | |
| C�� | Zn2+��Ca2+һ�������� | |
| D�� | 100mL��Һ��K+��������С��2.34g |
| A�� | ��Һ���ܼ�Fe2+ | B�� | ��Һ���ܼ�I2 | ||
| C�� | ��Һ���ܼ�Fe3+ | D�� | ��Һ���ܼ�Cl- |