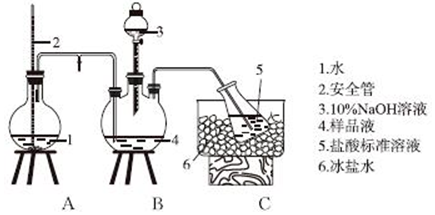
��Ŀ����
5����������Ҫ�Ļ�������ԭ��֮һ���ǻ�ѧ��ҵ������Ҫ�IJ�Ʒ�����ڽ������һֱ�ڸ�λ���У����ҹ���Ҫ�Ի�����Ϊԭ�������ᣮ���Ṥҵ��������β�����˺���N2��O2�⣬������SO2������SO3��������Ϊ�˱���������ͬʱ����ۺϾ���Ч�棬Ӧ�����ܽ�β���е�SO2ת��Ϊ���õĸ���Ʒ���밴Ҫ��ش��������⣺��1����β��ͨ�백ˮ�У��ᷢ�������Ӧ��д�����ܷ���������������ԭ��Ӧ��������ѧ����ʽ2��NH4��2SO3+O2�T2��NH4��2SO4��2NH4HSO3+O2�T2NH4HSO4��
��2����β���백ˮ��Ӧ���õ��ĸ�Ũ����Һ�У���һ���������백ˮ��̼����泥���ʱ��Һ���¶Ȼ����н��ͣ����������壮
�������ľ����������ֽ��ҵ��Ҳ��������������ӰҺ����������֪�ýᾧˮ�������Է�������Ϊ134�����仯ѧʽΪ��NH4��2SO3•H2O��
��������������Ҫ����Һ�м��������ĶԱ����ӻ�Ա����������ʣ���Ŀ���Ƿ�ֹ������隣�������
��3�������ڲⶨ����β����SO2��������BC��������ĸ��
��A��NaOH��Һ����̪��Һ ��B��KMnO4��Һ��ϡH2SO4
��C����ˮ��������Һ ��D����ˮ��ʯ����Һ��
���� ��1�����������е���Ϊ+4�ۣ����л�ԭ�ԣ������е��������������ԣ�
��2�������ľ���Ϊ��NH4��2SO3��NH4HSO3�����е�һ�ִ����ɽᾧˮ�������ᰱ�е���Ϊ+4�ۣ��ױ������е�����������
��3��KMnO4��Һ��ϡH2SO4 ������������������ɫ��KMnO4��Һ��ɫ���������������������ԭ��Ӧ��������������ɫ��
��� �⣺��1����β��ͨ�백ˮ�У�β���е�SO2�백ˮ��Ӧ����ˮ����ʱ��SO2+NH3•H2O=NH4HSO3����ˮ����ʱ��SO2+2NH3•H2O=��NH4��2SO3+H2O�����ɵ�������Ļ��ϼ�Ϊ+4�ۣ������������������ߵ�+6�ۣ�
�ʴ�Ϊ��2��NH4��2SO3+O2�T2��NH4��2SO4��2NH4HSO3+O2�T2NH4HSO4��
��2������β���백ˮ��Ӧ���õ��ĸ�Ũ����Һ�У���һ���������백ˮ��̼����泥���ˮ�������������NH4HSO3��������̼����立�Ӧ����Ϊ����������Ա�̼��ǿ���ʸýᾧˮ����Ϊ��NH4��2SO3�����ɽᾧˮ����NH4��2SO3��ʽ��Ϊ116����֪�ýᾧˮ�������Է�������Ϊ134��134-116=18���������к���1���ᾧˮ����ѧʽΪ��NH4��2SO3•H2O��
�ʴ�Ϊ����NH4��2SO3•H2O��
�ڶԱ����Ӿ��л�ԭ�ԣ������е��������������ԣ������ᰱ��+4�۵�����л�ԭ�ԣ�
�ʴ�Ϊ����ֹ������隣�������
��3��KMnO4��Һ��ϡH2SO4 ���������Ӧ�ķ���ʽΪ��5SO2+2MnO4-+2H2O=2Mn2++4H++5SO42-���������������������ԭ�ķ���ʽΪ��SO2+I2+2H2O=H2SO4+2HI���������꣬������û�еⵥ�ʲ�������ɫ��
�ʴ�Ϊ��BC��
���� ���⿼����������ԭ��Ӧ�����ӷ���ʽ����д�����ʵļ��顢Ԫ�ػ���������ʣ���Ŀ�Ѷ��еȣ������ڿ���ѧ���ķ��������ͶԻ���֪ʶ���ۺ�Ӧ��������ע��ӻ��ϼ۵ĽǶȷ���������ԭ��Ӧ��

| ѡ�� | ʵ����� | ���� | ���� |
| A | ��ϡ�����м�����������۳�ַ�Ӧ����KSCN��Һ | ��Һ��ΪѪ��ɫ | HNO3���������ԣ��ܽ�Fe������Fe3+ |
| B | ��ʢ��ij��Һ���Թ��еμ�NaOH��Һ����ʪ��ĺ�ɫʯ����ֽ�����Թܿ� | ��ֽ��ɫ�����Ա仯 | ԭ��Һ����NH4+ |
| C | �������Һ�м���ϡ���ᣬ���ȣ���ȴ���������Cu��OH��2���ټ��� | δ����ɫ���� | ����δ����ˮ�� |
| D | ���з�̪��Na2CO3��Һ�м�������BaCl2���� | ��Һ��ɫ��dz | ֤��Na2CO3��Һ�д���ˮ��ƽ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��״���£�3.36LC2H4��C3H6�Ļ�������к���̼̼˫������ĿΪ0.15NA | |
| B�� | 0.1mol•L-1��NH4��2SO4��Һ��0.2mol•L-1NH4Cl��Һ�е�NH4+��Ŀ��ͬ | |
| C�� | H2��CO�������8.96L������O2�г��ȼ������O2������Ϊ0.2NA | |
| D�� | ��0.1molNH4HSO4����Һ�У���������Ŀ�Դ���0.21NA |
�ֱ�ȡ���ǵ�ˮ��Һ����ʵ�飬������£�
| ������ | Na+ | Al3+ | Fe3+ | Cu2+ | Ba2+ |
| ������ | OH- | Cl- | CO32- | NO3- | SO42- |
��B ��Һ�� E ��Һ��Ϻ�������ɫ������ͬʱ�����������壻
������ C ��Һ�� D ��Һ��Ϻ������ɫ���������� C ��Һ�� D ��Һ��Ϻ�������
��B ��Һ�� D ��Һ��Ϻ�������
�ݽ� 38.4g Cu ƬͶ��װ������ D ��Һ���Թ��У�Cu Ƭ���ܽ⣬�ٵμ� 1.6mol/L ϡ H2SO4��Cu ���ܽ⣬�ܿڸ����к���ɫ������֣�
��1���ݴ��ƶ� A �Ļ�ѧʽΪ��ACuSO4��BFeCl3
��2��д������ C �� D ������Ӧ�����ӷ���ʽAl3++4OH-=AlO2-+2H2O��
��3��B ��Һ�е���ʯ����Һ����������Һ��죬ԭ����Fe3++3H2O?Fe��OH��3+3H+�������ӷ���ʽ˵����
�ٵ�һ�ݼ���AgNO3��Һ�г�������
�ڵڶ��ݼ�����BaCl2��Һ�ó�����6.63g������������ϴ�ӡ������������Ϊ4.66g����������Һ�м���AgNO3��Һ�г�������
��������ʵ�飬�����й�ԭ��Һ���۶ϲ���ȷ���ǣ�������
| A�� | Cl-һ������ | |
| B�� | CO32-��SO42-һ�����ڣ��Ҷ��ߵĸ�����Ϊ1��2 | |
| C�� | Zn2+��Ca2+һ�������� | |
| D�� | 100mL��Һ��K+��������С��2.34g |
| A�� | �۵㣺���ʯ���ɱ� | B�� | ���Ӱ뾶��O2-��Na+ | ||
| C�� | ���ԣ�KOH��Al��OH��3 | D�� | �ȶ��ԣ�SiH4��H2S |