��Ŀ����
4��ijѧϰС����ʵ�����о�SO2��Ba��NO3��2��Һ�ķ�Ӧ��ʵ�飺��ʢ��2mL 0.1mol/L Ba��NO3��2��Һ���Թ��У�����ͨ��SO2���壬�Թ����а�ɫ����������Һ���Ϸ�����dz��ɫ��
̽��1����ɫ����������ԭ��
��1����ɫ������BaSO4��
��2��������ɫ����������ԭ��ͬѧ��Ϊ��NO3-������SO2����ͬѧ��Ϊ����Һ���ܽ��O2������SO2��
��֧�ּ�ͬѧ�۵��ʵ��֤����Һ���Ϸ�����dz��ɫ��
�����ݼ��ƶϣ���д��Ba��NO3��2��Һ��SO2��Ӧ�����ӷ���ʽ3Ba2++2NO3-+3SO2+2H2O=3BaSO4��+2NO+4H+��
����ͬѧͨ������ʵ��֤�����Լ����Ʋ���ȷ�������ʵ�鷽����
| ʵ����� | ʵ������ |
��2mL0.1mol/LBaCl2 ��Һ���ѧʽ����ͨ��SO2 | �Թ����а�ɫ�������� |
| ʵ����� | ʵ������ |
| ���ձ��м�������˵�0.1mol/L��BaCl2��Һ25mL���ټ���25mLֲ���ͣ���ȴ�����£���pH�������ⶨ��ҺpH��ʱ�䣨t���ı仯���� |  ͼ1����BaCl2����������Һ��ͨ��SO2 |
| ���ձ��зֱ����25mL 0.1mol/L��BaCl2��Һ��Ba��NO3��2��Һ��ͨ��SO2����pH�������ֱ�ⶨ��ҺpH��ʱ�䣨t���仯�����ߣ� |  ͼ2���ֱ���BaCl2��Ba��NO3��2��Һ��ͨ��SO2 |
��4��ͼ2��BaCl2��Һ�з�����Ӧ�����ӷ���ʽΪ2Ba2++2SO2+O2+2H2O=2BaSO4��+4H+��
��5����������ͼ����ó��Ľ�����������SO2�Ĺ����У�O2������Ҫ���ã�˵��������BaCl2��Һ�����������õ���O2����Ba��NO3��2��Һ�����������õ���O2��NO3-����ͼ2�У��ֱ���BaCl2��Ba��NO3��2��Һ��ͨ��SO2��pH�仯���ơ����Ƚӽ���������������õ���Ҫ��O2��
���� ��1��SO2��Ba��NO3��2��Һ�ķ�Ӧ������Ԫ���غ��֪����ɫ����ӦΪBaSO4��
��2����NO3-������SO2�������������ԭ��һ��������һ�����������ٱ������ɶ���������ʹ��Һ���Ϸ�����dz��ɫ��
��Ba��NO3��2��Һ��SO2��Ӧ�������ᱵ��һ������������Ԫ���غ�͵���غ���д���ӷ���ʽ��
����ͬѧҪ֤�����Լ����Ʋ���ȷ�������Լ�Ӧ���ͬѧһ���������ſ������Աȣ���Ӧ����������ᱵ������
��3��������������ˮ���������ᣬ�������ʹ��pHֵ�½���
��4��ͼ2��BaCl2��Һ��ͨ�����������ͼ1��������������ӣ�˵�������μ��˷�Ӧ�����������ᱵ�����
��5����BaCl2��Һ�����������õ���O2����Ba��NO3��2��Һ�����������õ���O2��NO3-����ͼ2�У��ֱ���BaCl2��Ba��NO3��2��Һ��ͨ��SO2��pH�仯���ơ����Ƚӽ����ݴ˴��⣮
��� �⣺��1��SO2��Ba��NO3��2��Һ�ķ�Ӧ������Ԫ���غ��֪����ɫ����ӦΪBaSO4���ʴ�Ϊ��BaSO4��
��2����NO3-������SO2�������������ԭ��һ��������һ�����������ٱ������ɶ���������ʹ��Һ���Ϸ�����dz��ɫ������֧�ּ�ͬѧ�۵��ʵ��֤����Һ���Ϸ�����dz��ɫ��
�ʴ�Ϊ��Һ���Ϸ�����dz��ɫ��
��Ba��NO3��2��Һ��SO2��Ӧ�������ᱵ��һ����������Ӧ�����ӷ���ʽΪ3Ba2++2NO3-+3SO2+2H2O=3BaSO4��+2NO+4H+��
�ʴ�Ϊ��3Ba2++2NO3-+3SO2+2H2O=3BaSO4��+2NO+4H+��
����ͬѧҪ֤�����Լ����Ʋ���ȷ�������Լ�Ӧ���ͬѧһ���������ſ������Աȣ����������Լ�Ϊ0.1mol/LBaCl2��Һ�����Կ����а�ɫ�������֣�
�ʴ�Ϊ��0.1��BaCl2���Թ����а�ɫ����������
��3�������������£�������������ˮ���������ᣬ�������ʹ��pHֵ�½�����Ӧ�ķ���ʽΪSO2+H2O?H2SO3��H2SO3?HSO3-+H+��
�ʴ�Ϊ��SO2+H2O?H2SO3��H2SO3?HSO3-+H+��
��4��ͼ2��BaCl2��Һ��ͨ�����������ͼ1��������������ӣ�˵�������μ��˷�Ӧ�����������ᱵ�����ᣬ��Ӧ�����ӷ���ʽΪ2Ba2++2SO2+O2+2H2O=2BaSO4��+4H+��
�ʴ�Ϊ��2Ba2++2SO2+O2+2H2O=2BaSO4��+4H+��
��5���Ƚ�ͼ1��ͼ2��֪��������SO2�Ĺ����У�O2������Ҫ���ã���Ϊ��BaCl2��Һ�����������õ���O2����Ba��NO3��2��Һ�����������õ���O2��NO3-����ͼ2�У��ֱ���BaCl2��Ba��NO3��2��Һ��ͨ��SO2��pH�仯���ơ����Ƚӽ���
�ʴ�Ϊ��������SO2�Ĺ����У�O2������Ҫ���ã���BaCl2��Һ�����������õ���O2����Ba��NO3��2��Һ�����������õ���O2��NO3-����ͼ2�У��ֱ���BaCl2��Ba��NO3��2��Һ��ͨ��SO2��pH�仯���ơ����Ƚӽ���������������õ���Ҫ��O2��
���� ���⿼��������ʵ�鷽������ƣ���Ŀ�Ѷ��еȣ���ȷʵ��Ŀ�ļ�ʵ��ԭ��Ϊ���ؼ��������ֿ���ѧ���ķ�����������������ѧʵ��������ע��������������ʵ�鷽�������������ԭ��

 53���ò�ϵ�д�
53���ò�ϵ�д���ȡ����Ӧ
����ȥ��Ӧ
�ۼӾ۷�Ӧ
��������Ӧ
�ݻ�ԭ��Ӧ��
| A�� | �ݢڢ� | B�� | �ܢڢ� | C�� | �ݢڢ� | D�� | �٢ڢ� |
| A�� | �����£���ˮ�е��ܽ�ȣ��Ҷ������Ҵ������� | |
| B�� | ��������ȷ���л�������еĹ����� | |
| C�� | �ú˴Ź��������ܹ�����CH3CH2CHO��CH3COCH3 | |
| D�� | �Ҷ��ᡢ��ȩ������ϩ���ɷ����ۺϷ�Ӧ |
| A�� | ����ϡ���ᷴӦ��2Fe+6H+��2Fe3++3H2�� | |
| B�� | �������廯����Һ��Ӧ��C12+2Br-��2C1-+Br2 | |
| C�� | ����������ϡ���ᷴӦ��SO42-+Ba2+��BaSO4�� | |
| D�� | �����̼��Ʒ�Ӧ��2H++CaCO3��Ca2++CO2��+H2O |
| A�� | 0.2mol | B�� | 0.6mol | C�� | 0.8mol | D�� | l.0mol |
 ��Ϊԭ�Ϻϳɣ�·�����£����ַ�Ӧ�����ԣ���
��Ϊԭ�Ϻϳɣ�·�����£����ַ�Ӧ�����ԣ���



 ��
��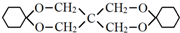 ��
�� ��
��
 2NH3��
2NH3��
