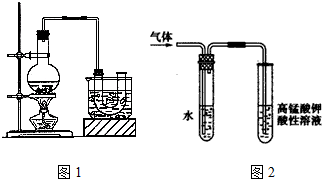
��Ŀ����
1���ⶨ����ͭ���壨CuSO4•xH2O���ᾧˮ������ʵ���������£�
�ش��������⣺
��1��������ʵ���п����õ��ļ�������������ͼ�·�������д���������ƣ�
a��
 ������ƽ b��
������ƽ b�� ���� c��
���� c�� ������ d��
������ d�� �ƾ����
�ƾ�����١�����I������������ͭ���壬�õ�����bd������ţ�������
�ڡ������IJ�������ȴ��������c������ţ��н��У�
��2�����ظ���������Ϊ���ز�����
�жϴﵽ���ص��������������γ�����������С��0.001g��
���к��ز�����Ŀ����ȷ������ʧȥȫ���ᾧˮ��
��3��ijѧ��ʵ���õ��Ա����ݣ�
| ����ǰ���� | ���Ⱥ����� | |
| m1�������� | m2������+���壩 | m3������+��ˮ����ͭ�� |
| 5.200g | 7.900g | 6.900g |
�ڸ�ѧ���ⶨ���ƫ�ߣ��ƫ�ߡ�����ƫ�͡�����ȷ������
�۴����з�����ѡ����ѧ������ʵ������ԭ������ǣ�����ţ�c��
a��û�н��к��ز���
b�����ȹ��嵽��ɫ��¶���ڿ�������ȴ
c�����ȹ����о�����������ʧ
d�����Ⱥ��ڸ���������ȴ�����º������
���� �ⶨ����ͭ���壨CuSO4•xH2O���нᾧˮ������ԭ��Ϊ�Ƚ���������в�����ϸ��Ȼ����������м��ȣ�ֱ��ǰ�����γ��������������0.001g��ֹͣ���ȣ�˵����ȫʧˮ���ٷ��ڸ���������ȴ��������ü���ǰ���������������ᾧˮ������
��1����������ͼ�ο����ж�bΪ����cΪ��������
�١�����I������������ͭ���壬���������м��ȣ�
�ڸ��ݲⶨ����ͭ���壨CuSO4•xH2O���нᾧˮ������ԭ�����������IJ����Ƿ��ڸ���������ȴ��
��2�����ݲⶨ����ͭ���壨CuSO4•xH2O���нᾧˮ������ԭ����ǰ�����γ��������������0.001g������Ϊ�ﵽ���أ�˵����ȫʧˮ��
��3���ٸ��ݽᾧˮ�����У��ᾧˮ������=m������ʮ���壩-m������ʮ��ˮ����ͭ�����ݻ�ѧ����ʽ���Լ���ᾧˮx��ֵ��
�ڸ��ݼ������xֵ������ֵ5�Ƚϣ�
���ڲⶨ����������Ʒ�к��м��Ȼӷ������ʻ�ʵ��ǰ��������ˮ��������ɲ������ƫ�ߡ�ƫ�ͣ��ݴ˷�����
��� �⣺��1����������ͼ�ο����ж�bΪ����cΪ���������ʴ�Ϊ����������������
�١�����I������������ͭ���壬���������м��ȣ������õ�����b������d�ƾ������������ѡ��bd��
�ڸ��ݲⶨ����ͭ���壨CuSO4•xH2O���нᾧˮ������ԭ�����������IJ����Ƿ��ڸ���������ȴ�����ԡ������IJ�������ȴ��������c�������н��У��ʴ�Ϊ����ȴ��c��
��2�����ݲⶨ����ͭ���壨CuSO4•xH2O���нᾧˮ������ԭ����ǰ�����γ��������������0.001g������Ϊ�ﵽ���أ�˵����ȫʧˮ�����жϴﵽ���ص��������������γ�����������С��0.001g�����к��ز�����Ŀ���� ȷ������ʧȥȫ���ᾧˮ���ʴ�Ϊ���������γ�����������С��0.001g��ȷ������ʧȥȫ���ᾧˮ��
��3�����ɱ����е����ݣ�����ͭ���������Ϊ7.900g-5.200g=2.700g��ˮ��������7.900g-6.900g=1.000g��
��CuSO 4��xH2O$\frac{\underline{\;\;��\;\;}}{\;}$CuSO 4+xH2O��
160+18x 18x
2.700g 1.000g
$\frac{160+18x}{2.700}$=$\frac{18x}{1.000}$�����x��5.2��
�ʴ�Ϊ��5.2��
���ɢټ����x��5.2�����������ֵ5�����Ը�ѧ���ⶨ���ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
��a����ͬѧδ�����ز������ᵼ�²ⶨ������ͭ������ƫ�ⶨ��ˮ������ƫС����a����
b�����ȹ��嵽��ɫ��¶���ڿ�������ȴ��������ͭ����ˮ���ᵼ�²ⶨ������ͭ������ƫ�ⶨ��ˮ������ƫС����b����
c�����ȹ��������������彦�����ᵼ�²ⶨ������ͭ������ƫС������ˮ�������ⶨ���ƫ��c��ȷ��
d�����Ⱥ��ڸ���������ȴ�����º������Ϊ��ȷ��������Ӱ�죬��d����
��ѡ��c��
���� ���⿼����ʵ���Ҳⶨ����ͭ����ᾧˮ����ʵ�飬��ʧˮ�õ�����ķ����ж��Լ���ʵ��������������ԭ����з����ǽ��Ĺؼ�����Ŀ�Ѷ��еȣ�

 ������ȫ��������ϵ�д�
������ȫ��������ϵ�д� ��������ƣ�Na2S2O3�������������ƺ����ͨ�����Ϸ�Ӧ�Ƶã���֪��Na2S2O3��������Һ�в����ȶ����ڣ�
��������ƣ�Na2S2O3�������������ƺ����ͨ�����Ϸ�Ӧ�Ƶã���֪��Na2S2O3��������Һ�в����ȶ����ڣ���1��ij�о�С��������Ʊ�Na2S2O3•5H2O��װ�úͲ��ֲ����������£�
��Kl������K2����Բ����ƿ�м�������Ũ���ᣬ���ȣ�
��C�еĻ��Һ��������������Ӧһ��ʱ�����۵������٣���C����Һ��pH�ӽ�7ʱ��ֹͣC�еķ�Ӧ��
����C�еĻ��Һ��
��������Һ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ���ɣ��õ���Ʒ��
�٢��У�Բ����ƿ�з�����Ӧ�Ļ�ѧ����ʽ�ǣ�Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
�ڢ��С�ֹͣC�еķ�Ӧ���IJ����Ǵ�K2���ر�K1��
�ۢ��н���Һ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ���ɣ��õ���Ʒ��Na2S2O3•5H2O���ܽ�����¶����������������ò�Ʒͨ���ؽᾧ�����ᴿ��
��װ��B����������C�еķ�Ӧֹͣ������A�в����Ķ���SO2��ֹ������Ⱦ��
��2�����ݷ�Ӧ2S2O32-+I2�TS4O62-+2I-������I2�ı���Һ�ⶨ��Ʒ�Ĵ��ȣ�ȡ5.5g��Ʒ�����Ƴ�100mL��Һ��ȡ10mL��Һ���Ե�����ҺΪָʾ������Ũ��Ϊ0.050mol•L-1I2�ı���Һ���еζ���������ݼ�¼���±���ʾ��
| ��� | 1 | 2 | 3 | 4 |
| ��Һ�����/mL | 10.00 | 10.00 | 10.00 | 10.00 |
| ����I2����Һ�����/mL | 19.99 | 19.98 | 17.13 | 20.03 |
��Na2S2O3•5H2O�ڲ�Ʒ�е�����������90.2%��Na2S2O3•5H2O��ʽ��Ϊ248������������1λС������
��1��ʵ�������÷�ӦTiO2��s��+2CCl4��g���TTiCl4��g��+CO2��g��������ˮ���������£���ȡTiCl4ʵ��װ��ʾ��ͼ����
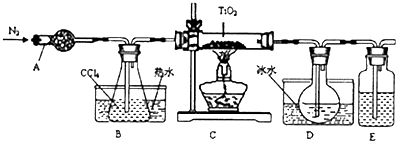
����������
| ���� | �۵�/�� | �е�/�� | ���� |
| CCl4 | -23 | 76 | ��TiCl4���� |
| TiCl4 | -25 | 136 | ����ʪ������������ |
��2����Ӧ���������ν������²�������Ϩ��ƾ��Ƣ�ֹͣͨ��������ȴ�����£���ȷ��˳��Ϊ�٢ۢڣ�����ţ���
��3��D�е�Һ̬�����ɷ���CCl4��TiCl4��Ҫ�������������ò���������������

| ���� | �۵� | �е� | ˮ���� | ��Է������� |
| �Ҷ��� | -12.9�� | 197.3�� | ����ˮ���� | 62 |
| �״� | -97�� | 64.7�� | ��ˮ���� | 32 |
| ��������� | 54�� | 163.5�� | ������ˮ�����ڴ����� | 118 |
ʵ������ͼ1��ʾװ��ģ�������������Ʊ���
����1������������ƿ�м���״���Ȼ��ͨ��A�ڽ���������ʱ��������Ũ���ᣬ���Һ��ȴ���ټ�����
����2����A��Ϊ���������ܣ���3�������¶ȼƣ���һ�����¶��»���2-3Сʱ��
����3�������Һ��������õ������������
�������Ҷ�������ȡ
ʵ������ͼ2��ʾ��װ��ģ�ҵ�Ҷ�������ȡ���г��豸�Ͳ��ּ���װ��ʡ�ԣ�
��Ӧ����ʽΪ��

�ش��������⣺
��1������A������Ϊ��Һ©��������3���������ǽ����������ˮ��ϣ���ϡ�����ȷ�����ǽ�������ر�������ע��װ����ˮ���ձ������������У������Ͻ��裮
��2��װ��B��������ʹ�����������������Ͼ��ȣ��ձ�C��ˮ���¶Ȳ��ܵ���55�棬ԭ�����¶ȵ���55�棬δ��Ӧ�IJ��������������������ܣ�
��3���Դֲ�Ʒ����Ҫ���Ҷ���������������ͼ״������о��ƣ������ռ�197.3�棨197�����Ҽ��ɣ������֣�
��4��ʵ������У�ʹ����16g H2��59g��������������õ��Ҷ���Ϊ12.4g���Ҷ����IJ���Ϊ40%��
��5�����ʵ��֤���Ҷ���Ϊ��Ԫ����ȡ62g�Ҷ������������������Ƴ�ַ�Ӧ���ռ����������ɵ�������������2g�������������𰸣��Ҷ�������������������ȷ�������ɣ���