��Ŀ����
16�� ij�о���ѧϰС��̽���������Һ���������������ʵ�飮
ij�о���ѧϰС��̽���������Һ���������������ʵ�飮��1��ȡһ�����ı���������250mL 0.5000mol•L-1������Һʱ��Ҫ�õ��IJ�����������Ͳ���ձ�����������250mL����ƿ�ͽ�ͷ�ιܣ�
��2��������0.5000mol•L-1�Ĵ�����Һ�ٽ���ϡ�ͣ�Ϊ�ⶨϡ�ͺ������Һ��ȷŨ�ȣ���0.2000mol•L-1��NaOH��Һ��25.00mL������Һ���еζ������εζ�����NaOH��Һ��������£�
| ʵ����� | 1 | 2 | 3 | 4 |
| ����NaOH��Һ�������mL�� | 25.05 | 25.00 | 23.80 | 24.95 |
��3��ʵ�飨2���У��ζ�������pH�仯������ͼ��ʾ�������£���
�ٵζ������У����μ�12.50mLNaOHʱ�����û����Һ������Ũ���ɴ�С˳��Ϊc��CH3COO-����c��Na+����c��H+����c��OH-����
�ڵ��μ�25.00mLNaOHʱ����Ӧ���û����Һ��pH=9��������Һ�У�ˮ�ĵ�����Ǵ�ˮ��100����
c��OH-��-c��CH3COOH��=10-9mol•L-1��
���� ��1����Ҫ�õ��IJ�����������Ͳ���ձ�����������250mL����ƿ�뽺ͷ�ιܣ�
��2����3��ʵ������NaOH��Һ���������3�����Ƚϴ�Ӧ����������3��ƽ��ֵΪ��������������Һ������ٽ��V��NaOH����c��NaOH��=V�����ᣩ��c�����ᣩ���㣻
��3���ٵ��μ�12.50mL NaOH����Һ�����ԣ��Ǵ���ʹ����ƣ���Һ�д������̶ȴ��ڴ����ˮ��̶ȣ�
�ڵ��μ�25.00mLNaOHʱ�������������ȣ�ǡ�÷�ӦΪCH3COONa��Ӧ����������������Ũ�ȼ��㣻
���������������غ㣺c��OH-��-c��CH3COOH��=c��H+����
��� �⣺��1������ȡ��ȡ���ᣬ���ձ����ܽ⣬���ò��������裬�ò�������������250mL����ƿ�У�����ý�ͷ�ιܶ��ݣ�
�ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ�
��2����3��ʵ������NaOH��Һ���������3�����Ƚϴ�Ӧ��������������������Һ���Ϊ25.00mL���ٽ��V��NaOH����c��NaOH��=V�����ᣩ��c�����ᣩ����֪c�����ᣩ=c��NaOH��=0.2000 mol•L-1��
�ʴ�Ϊ��0.2000 mol•L-1��
��3���ٵ��μ�12.50mL NaOH����Һ�����ԣ��Ǵ���ʹ����ƣ���Һ�д������̶ȴ��ڴ����ˮ��̶ȣ���Һ������Ũ�ȣ�c��CH3COO-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ��c��CH3COO-����c��Na+����c��H+����c��OH-����
�ڵ��μ�25.00mLNaOHʱ�������������ȣ�ǡ�÷�ӦΪCH3COONa��Ӧ����Һ������������Դ��ˮ�ĵ��룬������Һ��ˮ�ĵ�����Ǵ�ˮ��$\frac{\frac{1{0}^{-14}}{1{0}^{-9}}}{1{0}^{-7}}$=100����
���������������غ㣺c��OH-��-c��CH3COOH��=c��H+��=10-9mol/L��
�ʴ�Ϊ��100��10-9��
���� ���⿼��һ�����ʵ���Ũ����Һ���ơ��к͵ζ�������Ũ�ȴ�С�Ƚϵȣ�ע�����غ㡢�����غ㡢���Ӻ��ʽ������Ũ�ȵ�����ϵ�Ƚ���Ӧ�ã�

| A�� | ��pH��7ʱ����һ����C1V1��C2V2 | |
| B�� | ��pH��7ʱ�������Һ�п�����c��Na+����c��H+�� | |
| C�� | ��pH=7ʱ����V1=V2����һ����C2=C1 | |
| D�� | �� V1=V2��C1=C2����c��CH3COO-��+c��CH3COOH��=c��Na+�� |
| A�� | ��SO2ͨ��Ʒ����Һ����Һ��ɫ����Ȼָ�ԭɫ����SO2ͨ����ˮ����ˮ��ɫ�����Ҳ�ָܻ�ԭɫ | |
| B�� | �����������Ʊ�ʵ���У�����Na2CO3��Һ�������Խ��������������ܽ�ȣ��������ջӷ��������Ҵ������� | |
| C�� | ���к͵ζ���ʵ���У�����ƿ����ƿ������ˮϴ����ʹ�ã��ζ��ܺ���Һ��������ˮϴ������������ϴ��ʹ�� | |
| D�� | ��ȥ����CO2�л��е�����SO2���ɽ������������ͨ��ʢ������KMnO4��Һ��Ũ�����ϴ��ƿ |
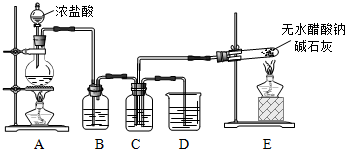
��1��װ��E�з�Ӧ����ʽ��CH3COONa+NaOH$��_{��}^{CaO}$Na2CO3+X����X�Ļ�ѧʽ��CH4��
��2����C���ռ�����X��Cl2Ϊ1��1�����ϣ�Ȼ���ڹ����·�Ӧ��
��Bװ�õ������dz�ȥCl2�к��е�HCl��
��Cװ����ʢ���Լ��DZ���ʳ��ˮ�����պ����ò�����CH3Cl��CH2Cl2��CHCl3��CCl4��HCl��
��3�����һ��ʵ�鷽������֤������������һ��������պ�ȡC��D��Һ����������ֱ������֧�Թ��У��ٷֱ�μ�2��ʯ����Һ��C����Һ��ɫ�������pH��ֽ�ⶨC��D����Һ��pH��
��4��ijѧ��Ϊ�˲ⶨX����ɽ���ʵ��̽����ѡ��������������
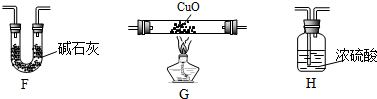
�ٸ�ͬѧ�������ӵĺ���˳����E��G��H��F��
�ڸ�ͬѧ�ⶨ����������ǣ�
| װ�� | ʵ��ǰ������/g | ʵ��������/g |
| F | 22.5 | 33.6 |
| H | 42.4 | 51.4 |
�òⶨ�������Ƿ���ƫ�����ƫ���˵��ԭ��F�����յ���CO2��CO2����33.6g-22.5g=11.1g��n��C��=$\frac{11.1g}{44g/mol}$=0.252mol��H�����յ���ˮ������H2O����51.4g-42.4g=9.0g��n��H��=$\frac{9.0g}{18g/mol}$��2=1.0mol��
��n��C����n��H����1��4��ԭ����Fװ�ú���������Ӵ��������е�ˮ������CO2������Bװ�ã���ɺ�̼��ƫ��
| A�� | ���뾶��K+��Al3+��S2-��Cl- | B�� | ���ӵĻ�ԭ�ԣ�S2-��Cl-��Br-��I- | ||
| C�� | ���ԣ�HClO��H2SO4��H3PO4��H2CO3 | D�� | �����ԣ�K��Ca��Mg��Be |
 �����ڸ�ԭ��˵����ȷ���ǣ�������
�����ڸ�ԭ��˵����ȷ���ǣ�������| A�� | �˵����Ϊ32 | B�� | ���������Ϊ6 | ||
| C�� | ��3�����Ӳ� | D�� | ���������Ӷ�2������ |
| A�� | ������ | B�� | �ʼ��� | C�� | ������ | D�� | $\frac{c��O{H}^{-}��}{c��{H}^{+}��}$=1 |
| A�� | ����ת��Ϊ���ף����������仯 | |
| B�� | ʯī���硢���ʯ�����磬�ʶ��߲���ͬ�������� | |
| C�� | O2��O3����ʽ��ͬ���ṹ��ͬ | |
| D�� | ��������S2��S4��S6�ȣ����Ƕ������ͬ�������� |
| A�� | x+10 | B�� | x+12 | C�� | x+16 | D�� | x+50 |