��Ŀ����
11��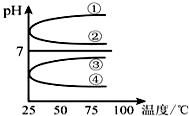 ��1��ϡ��0.1mol•L-1��ˮʱ������ˮ�������Ӷ���С���Ǣ٢ڣ���д��ţ���
��1��ϡ��0.1mol•L-1��ˮʱ������ˮ�������Ӷ���С���Ǣ٢ڣ���д��ţ�����$\frac{c��N{H}_{3}•{H}_{2}O��}{c��O{H}^{-}��}$ ��$\frac{c��O{H}^{-}��}{c��{H}^{+}��}$ ��c��H+����c��OH-���ij˻� ��OH-�����ʵ���
��2��pH��ͬ�ĵ������A��B������Һ��AΪ���ᣬBΪ���ᣩ�ֱ���п�۷�Ӧ����������һ����Һ�д���п�ۣ��ҷų�������������ͬ��������˵����ȷ���Ǣۢܢݣ���д��ţ���
�ٷ�Ӧ�����ʱ��B��A
�ڿ�ʼ��Ӧʱ������A��B
�۲μӷ�Ӧ��п�����ʵ���A=B
�ܷ�Ӧ�����е�ƽ������B��A
��A��Һ����п��ʣ��
��B��Һ����п��ʣ��
��3����������������ʵ���Ũ�ȵİ�ˮ�������Ϻ������¶ȣ����ʲ���ֽ⣩��Һ��pH���¶ȱ仯��ͼ�еĢ����ߣ���д��ţ���
��4�������£���0.1mol•L-1��������0.2mol•L-1�İ�ˮ�������ϣ���������Һ�������������ʵ���Ũ���ɴ�С��˳��Ϊc��NH4+����c��Cl-����c��OH-����c��H+����
��5���ɵ��볣����֪����ǿ����CH3COOH��H2CO3��HCO3-����Ũ����ͬ��������Һ������ǿ����˳��Ϊ���ۢڢ٣�����ţ�
��CH3COONa ��NaHCO3 ��Na2CO3
��6���Ȼ�����Һ�����Ե�ԭ���ǣ������ӷ���ʽ��ʾ����Fe3++3H2O?Fe��OH��3+3H+��
ij��Ԫ�ᣨ��ѧʽ��H2B��ʾ����ˮ�еĵ��뷽��ʽ�ǣ�H2B=H++HB-��HB-?H++B2-
����������⣺��0.1mol/L ��Na2B��Һ�У���������Ũ�ȹ�ϵʽ��ȷ����C��
A��c��B2-��+c��HB-��+c��H2B��=0.1mol/L
B��c��Na+��+c��OH-��=c��H+��+c��HB-��
C��c��Na+��+c��H+��=c��OH-��+c��HB-��+2c��B2-��
D��2c��Na+��=c��B2-��+c��HB-��
���� ��1����ˮϡ�ʹٽ�һˮ�ϰ����룬��Һ��c��OH-����c��NH3��H2O����c��NH4+������С���¶Ȳ��䣬ˮ�����ӻ��������䣬��c��H+������
��2��pH��ͬ�ĵ������A��B������Һ��AΪ���ᣬBΪ���ᣩ�ֱ���п�۷�Ӧ����������һ����Һ�д���п�ۣ��ҷų�������������ͬ�����ڴ��Ჿ�ֵ��룬�����Ũ�ȴ���������Ũ�ȣ�������HCl��Ũ�ȵ��������ӵ�Ũ�ȣ����Դ����Ũ�ȴ���HCl��Ũ�ȣ���������п��ʣ�ࣻ
��3��������������ʵ���Ũ�ȵİ�ˮ�������Ϻ�ǡ�������Ȼ����Һ��笠�����ˮ�������ԣ����ȴٽ�ˮ�⣻
��4�������£���0.1mol•L-1��������0.2mol•L-1�İ�ˮ�������ϵõ���ҺΪ��Ũ�ȵ��Ȼ�狀�һˮ�ϰ��Ļ����Һ��һˮ�ϰ��������笠�����ˮ�⣬��Һ�Լ��ԣ�
��5�������ε�ˮ����ɣ�Խ��Խˮ����������ˮ���ԭ�����ش�
��6���Ȼ���Ϊǿ�������Σ�������ˮ�����Һ�����ԣ��κε������Һ�ж����ڵ���غ�������غ㣬�ݴ˷������
��� �⣺��1����ˮϡ�ʹٽ�һˮ�ϰ����룬��Һ������������Ũ�ȡ�һˮ�ϰ�Ũ�ȡ�笠�����Ũ�ȶ���С����c��H+������
�٣���ˮϡ�ʹٽ�һˮ�ϰ����룬������������Ŀ����һˮ�ϰ���������С��ͬ��Һ��������䣬��$\frac{c��N{H}_{3}•{H}_{2}O��}{c��O{H}^{-}��}$��С���ʢ���ȷ��
�ڣ���ˮϡ�ʹٽ�һˮ�ϰ����룬����������Ũ�ȼ�С���¶Ȳ��䣬ˮ�����ӻ��������䣬��������Ũ����������$\frac{c��O{H}^{-}��}{c��{H}^{+}��}$ ��С���ʢ���ȷ��
�ۣ��¶Ȳ��䣬ˮ�����ӻ��������䣬����c��H+����c��OH-���ij˻����䣬�ʢ۴���
�ܣ���ˮϡ�ʹٽ�һˮ�ϰ����룬��OH-�����ʵ������ʢܴ���
�ʴ�Ϊ���٢ڣ�
��2��pH��ͬ�ĵ������A��B������Һ��AΪ���ᣬBΪ���ᣩ�ֱ���п�۷�Ӧ����������һ����Һ�д���п�ۣ��ҷų�������������ͬ�����ڴ��Ჿ�ֵ��룬�����Ũ�ȴ���������Ũ�ȣ�������HCl��Ũ�ȵ��������ӵ�Ũ�ȣ����Դ����Ũ�ȴ���HCl��Ũ�ȣ���������п��ʣ�ࣻ
�����ڴ��������������Ӧ�Ͽ죬���Է�Ӧ�����ʱ��A��B���ʴ���
�ڿ�ʼpH��ͬ����������Ũ����ͬ�����Կ�ʼʱ��Ӧ����A=B���ʴ���
���������ɵ����������ͬ�����Բμӷ�Ӧ��п�����ʵ���A=B������ȷ��
�ܴ����Ũ�ȴ��������Ũ�ȣ�������з�Ӧ���ʴ����Է�Ӧ�����е�ƽ������ B��A������ȷ��
�ݴ����Ũ�ȴ��������Ũ�ȣ�������ʣ�࣬����������п��ʣ�࣬����ȷ��
�����Ũ�ȴ��������Ũ�ȣ�������ʣ�࣬����������п��ʣ�࣬�ʴ���
�ʴ�Ϊ���ۢܢݣ�
��3��������������ʵ���Ũ�ȵİ�ˮ�������Ϻ�ǡ�������Ȼ����Һ��NH4+ˮ����Һ�����ԣ�PH��7���٢�pH����7���ʢ٢ڴ���
���ȴٽ�ˮ�⣬����ˮ��ƽ�������ƶ���c��H+������������ǿ��PH��С���۴�����ȷ��
�ʴ�Ϊ���ܣ�
��4�������£���0.1mol•L-1��������0.2mol•L-1�İ�ˮ�������ϵõ���ҺΪ��Ũ�ȵ��Ȼ�狀�һˮ�ϰ��Ļ����Һ��һˮ�ϰ��������笠�����ˮ�⣬��Һ�Լ��ԣ���Һ������Ũ�ȴ�СΪ��c��NH4+����c��Cl-����c��OH-����c��H+����
�ʴ�Ϊ��c��NH4+����c��Cl-����c��OH-����c��H+����
��5���ɵ��볣����֪����ǿ����CH3COOH��H2CO3��HCO3-������ˮ��̶ȣ�̼������ӣ�̼���������������ӣ����Ԣ�CH3COONa ��NaHCO3����Na2CO3�ļ���˳���ǣ�
�ۣ��ڣ��٣��ʴ�Ϊ���ۣ��ڣ��٣�
��6���Ȼ���Ϊǿ�������Σ�������ˮ�����Һ�����ԣ�ˮ�ⷽ��ʽΪFe3++3H2O?Fe��OH��3+3H+��
A��H2B��һ����ȫ���룬������Һ�в�����H2B��Ӧ��Ϊc��B2-��+c��HB- ��=0.1mol/L����A����
B��H2B��һ����ȫ���룬������Һ�в�����H2B����Һ�д��������غ㣬���������غ��c��OH-��=c��H+��+c��HB-������B����
C�����ݵ���غ��c��Na+��+c��H+ ��=c��OH- ��+c��HB-��+2c��B2- ������C��ȷ��
D�����������غ��c��Na+��=2c��B2- ��+2c��HB- ������D����
�ʴ�Ϊ��Fe3++3H2O?Fe��OH��3+3H+��C��
���� ���⿼��������Һ֮��ķ�Ӧ���漰��ǿ����ʡ�������ʵĵ��롢�����ˮ�⼰��Һ��pHֵ������Ũ�ȴ�С�ıȽϡ�����ƽ�ⳣ���ļ����֪ʶ����Ŀ�漰��֪ʶ��϶࣬�ۺ��Խ�ǿ����Ŀ�Ѷ��еȣ�

 �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�| A�� | Al3+ | B�� | Fe2+ | C�� | Al | D�� | Br- |
| A�� | 150 mL 3 mol•L-1�������Һ | B�� | 75 mL 3 mol•L-1�Ȼ�����Һ | ||
| C�� | 150 mL 3 mol•L-1�Ȼ�����Һ | D�� | 50 mL 3 mol•L-1�Ȼ�þ��Һ |
| A�� | ��������ɱ����ۻ� | B�� | Һ���Һ�������� | ||
| C�� | ʳ�κͱ����ۻ� | D�� | ������ռ���ۻ� |
| A�� | �������ݻ�ѹ��һ�룬ƽ�������ƶ����ﵽ��ƽ��ʱc��H2����2mol•L-1 | |
| B�� | �������м���0.1molN2��ƽ�������ƶ����ﵽ��ƽ��ʱN2���������С��20% | |
| C�� | ���ﵽ��ƽ��ʱc��H2��=2.5mol•L-1����ı�����������ǽ��»��ѹ | |
| D�� | ���ı�������H2�İٷֺ���������ƽ��һ�����淴Ӧ�����ƶ� |
