��Ŀ����
3����֪A��B��C��D����ѧ��ѧ�������ʣ�������һ���������У�A+B��C+D��ת����ϵ����1����AΪ��������BΪ���������÷�Ӧ��һ����;�Ǻ��Ӹֹ죮
��2����A��һ����ʹʪ��ĺ�ɫʯ����ֽ���������壬�Ҹ÷�Ӧ�ǹ�ҵ����ȡ�������Ҫ��Ӧ֮һ���÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪ4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
��3����A�ǵ���ɫ��ĩ����������������CΪǿ���÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪ2Na2O2+2H2O=4NaOH+O2����
��4����A��B��D�����л����������A��B�Ǽ�ͥ�����г�����ζƷ����Ҫ�ɷ֣���A����Է���������B��14���÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪCH3COOH+C2H5OH$��_{��}^{ŨH_{2}SO_{4}}$CH3COOC2H5+H2O���䷴Ӧ����Ϊȡ������������Ӧ��
���� ��1����AΪ��������BΪ����������A+B��C+DΪ���ȷ�Ӧ��
��2����A��һ����ʹʪ��ĺ�ɫʯ����ֽ���������壬��Ϊ�������÷�Ӧ�ǹ�ҵ����ȡ�������Ҫ��Ӧ֮һ����÷�ӦΪ���Ĵ�������
��3����A�ǵ���ɫ��ĩ����������������CΪǿ���AΪ�������ƣ��÷�ӦΪ����������ˮ�ķ�Ӧ��
��4����A��B��D�����л����������A��B�Ǽ�ͥ�����г�����ζƷ����Ҫ�ɷ֣���A����Է���������B��14����AΪ���ᣬBΪ�Ҵ���DΪ�����������ݴ˴��⣮
��� �⣺��1����AΪ��������BΪ����������A+B��C+DΪ���ȷ�Ӧ���÷�Ӧ��һ����;�Ǻ��Ӹֹ죬�ʴ�Ϊ�����Ӹֹ죻
��2����A��һ����ʹʪ��ĺ�ɫʯ����ֽ���������壬��Ϊ�������÷�Ӧ�ǹ�ҵ����ȡ�������Ҫ��Ӧ֮һ����÷�ӦΪ���Ĵ���������Ӧ�Ļ�ѧ��Ӧ����ʽΪ4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
�ʴ�Ϊ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
��3����A�ǵ���ɫ��ĩ����������������CΪǿ���AΪ�������ƣ��÷�ӦΪ����������ˮ�ķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2H2O=4NaOH+O2����
�ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2����
��4����A��B��D�����л����������A��B�Ǽ�ͥ�����г�����ζƷ����Ҫ�ɷ֣���A����Է���������B��14����AΪ���ᣬBΪ�Ҵ���DΪ�����������÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪCH3COOH+C2H5OH$��_{��}^{ŨH_{2}SO_{4}}$ CH3COOC2H5+H2O���÷�ӦΪȡ������������Ӧ��
�ʴ�Ϊ��CH3COOH+C2H5OH$��_{��}^{ŨH_{2}SO_{4}}$ CH3COOC2H5+H2O��ȡ������������Ӧ��
���� ���⿼��������ת����ϵ���ƶϺ�Ӧ�ã��Ѷ��еȣ�����ʱע�ⳣ��Ԫ�ػ�����֪ʶ����������Լ��л�������֪ʶ��Ӧ�ã�

 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д�| A�� | ͬ��ͬѹ�£���ͬ��������ʣ����ǵ����ʵ���һ����� | |
| B�� | �κ������£������ʵ����Ķ��������һ����̼�����ķ�����һ����� | |
| C�� | 1Lһ����̼����һ����1L����������С | |
| D�� | ������������ʵ���Ũ�ȵ�ǿ����������H+��һ����� |
| A�� | һ�������£�6.4 gͭ���������Ӧ��ת�Ƶ�����ĿΪ0.2NA | |
| B�� | 3mol����Fe��ȫת��ΪFe3O4��ʧȥ8NA������ | |
| C�� | ��״���£�11.2L SO3 �к���2NA��ԭ�� | |
| D�� | ��ʯ������ȫ����1 mol Cl2ʱ��ת�Ƶ��ӵ���Ŀ��2NA |
| A�� | ����Mg�ܷų�H2����Һ�У�K+��Al3+��Cl-��SO42- | |
| B�� | ���ڽ϶��Fe3+����Һ�У�HCO3-��Cl-��SO42- | |
| C�� | ˮ���������c��OH-��=1��10-10 mol/L����Һ�У�Al3+��SO42-��NO3-��Cl- | |
| D�� | ʹ���ȱ��ɫ����Һ�У�Na+��AlO2-��NO3-��CO32- |
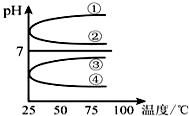 ��1��ϡ��0.1mol•L-1��ˮʱ������ˮ�������Ӷ���С���Ǣ٢ڣ���д��ţ���
��1��ϡ��0.1mol•L-1��ˮʱ������ˮ�������Ӷ���С���Ǣ٢ڣ���д��ţ��� ��
��  D��35Cl��37Cl
D��35Cl��37Cl
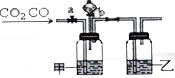 ijѧ������ͼװ�ý���CO��CO2�������ķ���������aΪ���ɼУ���������ͨ������bΪ��Һ©���Ļ������û������������ڿ��Ʒ�Һ©����Һ����������
ijѧ������ͼװ�ý���CO��CO2�������ķ���������aΪ���ɼУ���������ͨ������bΪ��Һ©���Ļ������û������������ڿ��Ʒ�Һ©����Һ����������