��Ŀ����
20��ij�о���ѧϰС��Ϊ̽��ijҽҩ��˾��Ʒ��Һ�岹Ѫ���е���Ԫ�أ�����������ʵ�飺���Թ��м���Һ�岹����2mL����������ˮ��������Һ����������μ�KSCN��Һ����Һ��ʾ����ɫ�������õĵ���ɫ��Һ�ֳ����ݼ�������ʵ��
�Իش��������⣺
��1����Ѫ����Һ�еμ�KSCN��Һ����ʾ����ɫ��ԭ�����������������ӱ�������
��2����д������ٶ�Ӧ�����ӷ���ʽFe+2Fe3+�T3Fe2+��
��д������ڶ�Ӧ�����ӷ���ʽ2Fe2++H2O2+2H+�T2Fe3++2H2O��
��3����֪�����ԣ�Br2��Fe3+��I2������ɫ��Һ�ֳ����ݣ��ֱ�μ���ˮ����ˮ��ʵ������ֱ�Ϊ������ˮ��Һ��Ѫ��ɫ���ӵ�ˮ����������
��4�����ڢ��е�ʵ������ͬѧ����˼��裺������˫��ˮ��SCN-�����ˣ��������һ��ʵ�鷽����֤���ļ���ȡ�����������˫��ˮ�����Һ�������м������KSCN��Һ������Һ�����˵���ǹ�����˫��ˮ��SCN-�����ˣ�
���� ��1������Fe2+��������Fe3+��Fe3+��SCN-��죻
��2������ټ������������ۣ����Ի�ԭ��Һ�е������ӣ������������ӣ�������м�˫��ˮ������Һ�е��������������������ӣ�
��3������ɫ��Һ�м��������������������ӣ�������ˮ���������������������������ӣ������ˮ��û�з�Ӧ������
��4��������Ӧ�����Һ�����¼������軯����Һ�����������жϱ��ļ���ĺ����ԣ�
��� �⣺��1��Fe2+�ױ���������Fe3+��Fe3+��SCN-����������ʹ��Һ���ɫ����Ϊ��Ѫ���в����������ӱ��������������ӣ�������Ѫ����Һ�еμ�KSCN��Һ����Һ��ʾ����ɫ��
�ʴ�Ϊ�����������������ӱ�������
��2������ټ������������ۣ����Ի�ԭ��Һ�е������ӣ������������ӣ���Ӧ�����ӷ���ʽΪFe+2Fe3+�T3Fe2+��������м�˫��ˮ������Һ�е��������������������ӣ���Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+�T2Fe3++2H2O��
�ʴ�Ϊ��Fe+2Fe3+�T3Fe2+��2Fe2++H2O2+2H+�T2Fe3++2H2O��
��3������ɫ��Һ�м��������������������ӣ�������ˮ���������������������������ӣ�������Һ��ΪѪ��ɫ�������ˮ��û�з�Ӧ��������������������
�ʴ�Ϊ������ˮ��Һ��Ѫ��ɫ���ӵ�ˮ����������
��4��������Ӧ�����Һ�����¼������軯����Һ�����������жϱ��ļ���ĺ����ԣ�ʵ�����Ϊȡ�����������˫��ˮ�����Һ�������м������KSCN��Һ������Һ�����˵���ǹ�����˫��ˮ��SCN-�����ˣ�
�ʴ�Ϊ��ȡ�����������˫��ˮ�����Һ�������м������KSCN��Һ������Һ�����˵���ǹ�����˫��ˮ��SCN-�����ˣ�
���� ���⿼����̽���������ơ���Ҫ�ɷ֣���ֿ�����ѧ���ķ�������������������ѧ֪ʶ����������������Ѷ��еȣ�

| A�� | CH3CH=CH2+Br2$\stackrel{CCl_{4}}{��}$CH3CHBrCH2Br | |
| B�� | CH3CH2OH $��_{170��}^{ŨH_{2}SO_{4}}$ CH2=CH2��+H2O | |
| C�� | CH3COOH+CH3CH2OH $��_{��}^{ŨH_{2}SO_{4}}$ CH3COOCH2CH3+H2O | |
| D�� | 2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O |
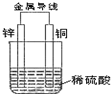
| A�� | ͭƬ�������ݲ��� | B�� | ͭƬ�������� | ||
| C�� | ���Ӵ�пƬ����������ͭ | D�� | ��װ�ðѻ�ѧ��ת��Ϊ���� |
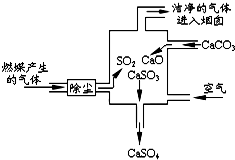
| A�� | �˹�����û�зֽⷴӦ | |
| B�� | �������̵ķ�Ӧ�ɱ�ʾΪ��2SO2+2CaCO3+O2�T2CaSO4+2CO2 | |
| C�� | ʹ�ô˷�������װ�ÿɼ���CO2���ŷ� | |
| D�� | �˹�����SԪ�صĻ��ϼ�δ�����ı� |
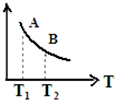
| A�� | ͬʱ���� | B�� | ͬʱ���� | C�� | v����������v���棩���� | D�� | v���������٣�v���棩���� |
| A�� | ���Ƶ���ˮ�ڹ�������ɫ��dz | |
| B�� | H2��I2��HIƽ��������С�����ѹ����ɫ���� | |
| C�� | ��ҵ����������Ĺ����У�SO2�ڴ�����ʱ��������Ϊ��ѹ�������Ǹ�ѹ | |
| D�� | ��ҵ�Ϻϳɰ���ʱ�¶�ѡ��450�����ң������dz��� |
 ��֪25��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ��
��֪25��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ��| ��ѧʽ | CH3COOH | H2CO3 | HClO | |
| ����ƽ�ⳣ�� | Ka=1.8��10-5 | Kal=4.3��10-7 | Ka2=5.6��10-11 | Ka=3.0��10-8 |
��1�����ʵ���Ũ�Ⱦ�Ϊ0.1mol•L-1��������Һ��a��Na2CO3 b��NaHCO3 c��NaClO d��CH3COONa�����ǵ�pH�ɴ�С���е�˳����a��c��b��d �����ţ���
��2�������£�0.1mol•L-1CH3COOH��Һ��ˮϡ�����У����б���ʽ�����ݱ�����BC��
A��c��H+�� B��$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$
C��$\frac{c��O{H}^{-}��}{c��{H}^{+}��}$ D��c��H+��•$\frac{c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}$
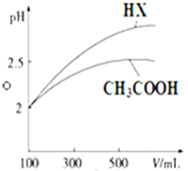
��3��CH3COOH��һԪ��HX����Һ��Ϊ100mL��pH=2����ˮϡ������pH����Һ����Ĺ�ϵ��ͼ��ʾ����ͬ�¶�ʱCH3COOH�ĵ���ƽ�ⳣ��С�ڣ�����ڡ�����С�ڡ����ڡ��� HX�ĵ���ƽ�ⳣ����
��4��25��ʱ��CH3COOH��CH3COONa�Ļ����Һ�������pH=6������Һ��c��CH3COO-��-c��Na+��=9.9��10-7 mol•L-1��������λ��Ч���֣���
 ̼�������仯�����ڹ�ũҵ����������������Ҫ���ã���ش��������⣺
̼�������仯�����ڹ�ũҵ����������������Ҫ���ã���ش��������⣺