��Ŀ����
18��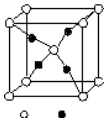 ���ڱ�ǰ�����ڵ�Ԫ��A��B��C��D��E��ԭ��������������A�ĺ����������������������ͬ��B�ļ۵��Ӳ���δ�ɶԵ�����3����C������������Ϊ���ڲ��������3����D��Cͬ�壻E�������ֻ��1�����ӣ����������18�����ӣ��ش��������⣻
���ڱ�ǰ�����ڵ�Ԫ��A��B��C��D��E��ԭ��������������A�ĺ����������������������ͬ��B�ļ۵��Ӳ���δ�ɶԵ�����3����C������������Ϊ���ڲ��������3����D��Cͬ�壻E�������ֻ��1�����ӣ����������18�����ӣ��ش��������⣻��1��B��C��D�е�һ������������N����Ԫ�ط��ţ���C�ĵ����Ų�ʽΪ1s22s22p4��
��2��A������Ԫ���γɵĶ�Ԫ���ۻ������У����ӳ������Σ��÷��ӵ�����ԭ�ӵ��ӻ���ʽΪsp3�������мȺ��м��Թ��ۼ����ֺ��зǼ��Թ��ۼ��Ļ�������H2O2��N2H4���ѧʽ����
��3����ЩԪ���γɵĺ������У����ӵ�����ԭ�ӵļ۲���Ӷ���Ϊ3������HNO2��HNO3������������ṹ������H2SO3�����ѧʽ��
��4��E��C�γɵ�һ�����ӻ�����ľ���ṹ��ͼ��a������E�����ӷ���ΪCu+��
���� ���ڱ�ǰ�����ڵ�Ԫ��A��B��C��D��E��ԭ��������������A�ĺ����������������������ͬ����A��HԪ�أ�C������������Ϊ���ڲ��������3����ԭ��������������8������C��OԪ�أ�D��Cͬ�壬��D��SԪ�أ�B�ļ۵��Ӳ��е�δ�ɶԵ�����3������ԭ������С��C����B��NԪ�أ�E�������ֻ��һ�����ӣ����������18�����ӣ���E��CuԪ�أ��ٽ��ԭ�ӽṹ�����ʽṹ��Ԫ�������ɽ��
��� �⣺��1���������Ϸ�����B��C��D�ֱ���N��O��SԪ���У�Ԫ�صķǽ�����Խǿ�����һ������Խ��ͬһ����Ԫ���У���һ����������ԭ��������������������ƣ�����VA��Ԫ�ش�������Ԫ�أ�����N��O��S�е�һ������������NԪ�أ�CΪOԪ�أ�������8�����ӣ������Ų�ʽΪ1s22s22p4��
�ʴ�Ϊ��N��1s22s22p4��
��2��A��HԪ�أ�A������Ԫ���γɵĶ�Ԫ���ۻ������У����ӳ������Σ��÷���Ϊ���������������е�ԭ�Ӻ���3�����ۼ���һ���µ��Ӷԣ����Ը÷��ӵ�����ԭ�ӵ��ӻ���ʽΪsp3�������мȺ��м��Թ��ۼ����ֺ��зǼ��Թ��ۼ��Ļ�������H2O2��N2H4��
�ʴ�Ϊ��sp3��H2O2��N2H4��
��3����ЩԪ���γɵĺ������У����ӵ�����ԭ�ӵļ۲���Ӷ���Ϊ3������HNO2��HNO3������������ṹ������H2SO3��
�ʴ�Ϊ��HNO2��HNO3��H2SO3��
��4��E��C�γɵ�һ�����ӻ�����ľ���ṹ��ͼ1��C���Ӹ���=1+8��$\frac{1}{8}$=2��E���Ӹ���=4�����Ըû�����ΪCu2O����E���ӷ���ΪCu+���ʴ�Ϊ��Cu+��
���� ���⿼���˾����ļ��㡢��ѧ�����жϡ������ܵıȽϵ�֪ʶ�㣬��Щ֪ʶ�㶼�Ǹ߿��ȵ㣬���ݾ����ص㡢��ѧ���Ĺ�������Ԫ�������ɵ�֪ʶ�������������Ŀ�Ѷ��еȣ�

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�| A�� | OH-�����ʵ��� | B�� | c��H+�� c��OH-�� | C�� | c��NH4+�� | D�� | c��OH-�� |
| A�� | ������ֱ���������Է�����3��̼ԭ��Ҳ��һ��ֱ���� | |
| B�� | ��ϩ����ԭ�Ӿ���ͬһƽ���� | |
| C�� | 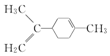 ����̼ԭ��һ����ͬһƽ���� ����̼ԭ��һ����ͬһƽ���� | |
| D�� |  ������16��ԭ�ӹ�ƽ�棬����8ԭ�ӹ��� ������16��ԭ�ӹ�ƽ�棬����8ԭ�ӹ��� |
| A�� | Fe��Cl2��Ӧ����FeCl3���Ʋ�Fe��I2��Ӧ����FeI3 | |
| B�� | CO2��Ba��NO3��2��Һ����Ӧ��SO2��Ba��NO3��2��ҺҲ����Ӧ | |
| C�� | CO2��ֱ���ͷ��ӣ��Ʋ�CS2Ҳ��ֱ���ͷ��� | |
| D�� | NaCl��ŨH2SO4���ȿ���HCl���Ʋ�NaBr��ŨH2SO4���ȿ���HBr |
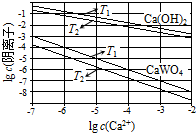 ��֪�������ƺ�����ƣ�CaWO4�������ܵ���ʣ����ߵ��ܽ�Ⱦ����¶����߶���С������ұ�������У����������Ƽ��������Ƽ�����Һ�еõ�����ƣ�������Ӧ
��֪�������ƺ�����ƣ�CaWO4�������ܵ���ʣ����ߵ��ܽ�Ⱦ����¶����߶���С������ұ�������У����������Ƽ��������Ƽ�����Һ�еõ�����ƣ�������Ӧ��WO42-��aq��+Ca��OH��2��s��?CaWO4��s��+2OH-��aq����
��1����ͼΪ��ͬ�¶���Ca��OH��2��CaWO4�ij����ܽ�ƽ�����ߣ�
�ټ���T1ʱKSP��CaWO4��=1��10-10��
��T1�� T2���������=����������
��2����Ӧ���ƽ�ⳣ��K����ֵ�����
| �¶�/�� | 25 | 50 | 90 | 100 |
| K | 79.96 | 208.06 | 222.88 | 258.05 |
�ڸ÷�Ӧ�ġ�H��0���������=����������
��3�����������ŨAlCl3����Һ�в���ͨ��HCl���壬����������AlCl3•6H2O���壬��ϻ�ѧƽ���ƶ�ԭ���������������ԭ��AlCl3������Һ�д����ܽ�ƽ�⣺AlCl3•6H2O��s���TAl3+��aq��+3Cl-��aq��+6H2O��l����ͨ��HCl����ʹ��Һ��c��Cl-������ƽ������������ķ����ƶ��Ӷ�����AlCl3•6H2O���壮
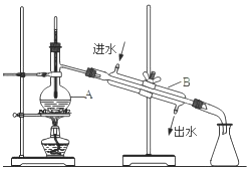 ��ͼΪʵ�����Ʊ�����ˮ��װ��ʾ��ͼ������ͼʾ�ش��������⣮
��ͼΪʵ�����Ʊ�����ˮ��װ��ʾ��ͼ������ͼʾ�ش��������⣮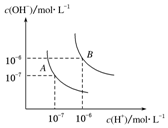 ˮ�ĵ���ƽ��������ͼ��ʾ����A���ʾ25��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�B���ʾ100��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ���100��ʱ1mol•L-1��NaOH��Һ�У���ˮ�������c��H+��=1��10-12mol•L-1��KW��25�棩��KW��100�棩�����������������=������
ˮ�ĵ���ƽ��������ͼ��ʾ����A���ʾ25��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�B���ʾ100��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ���100��ʱ1mol•L-1��NaOH��Һ�У���ˮ�������c��H+��=1��10-12mol•L-1��KW��25�棩��KW��100�棩�����������������=������