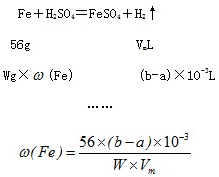��Ŀ����
����̼�����ƺ�̼���ƹ������Ϊ�˲ⶨ�������̼���Ƶİٷֺ�������������װ�ã�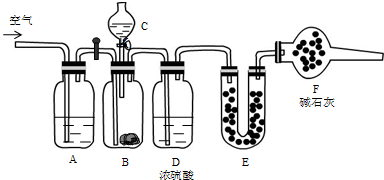
ʵ�鲽�裺
�ټ��װ�������Ԣڽ�ҩƷװ�ã�����B��װ�� 9.5g��Ʒ��Eװ��ҩƷ����������56.0g
�۹رջ���K����Һ©������������Һ�壬��ַ�Ӧ
�ܴ�B����ȫ��Ӧ����K��ͨ��һ�������
�ݳ���Eװ������Ϊ60.4g
�ش��������⣺
��1��C��װ��ҩƷ�� ��E��ҩƷ�� ��F��ҩƷ������ ��
��2��A�з�����Ӧ�����ӷ���ʽΪ ��
��3����Ʒ��̼���Ƶ������ٷֺ���Ϊ ��
��4����ʵ���к��ڲ�ͨ����������������Ʒ��̼���Ƶİٷֺ��� ���ƫС����ƫ����Ӱ�족��

ʵ�鲽�裺
�ټ��װ�������Ԣڽ�ҩƷװ�ã�����B��װ�� 9.5g��Ʒ��Eװ��ҩƷ����������56.0g
�۹رջ���K����Һ©������������Һ�壬��ַ�Ӧ
�ܴ�B����ȫ��Ӧ����K��ͨ��һ�������
�ݳ���Eװ������Ϊ60.4g
�ش��������⣺
��1��C��װ��ҩƷ��
��2��A�з�����Ӧ�����ӷ���ʽΪ
��3����Ʒ��̼���Ƶ������ٷֺ���Ϊ
��4����ʵ���к��ڲ�ͨ����������������Ʒ��̼���Ƶİٷֺ���
���㣺̽�����ʵ���ɻ�������ʵĺ���,�Ƶ���Ҫ������
ר�⣺������Ҫ�Ľ������仯����
��������1������װ��ͼ��֪��ԭ���������̼���η�Ӧ����CO2��Ȼ��ͨ������CO2�����㺬�������������ӷ�������C����ϡ���ᣬE���Ǽ�ʯ�ң����ڿ����к���CO2��ˮ����������F�м�ʯ�ҵ����������յ������������ˮ������������̼���壬��ֹ��E�����ĸ��ţ�
��2��A�����տ����е�CO2�ģ�����ʽΪCO2+2OH-=CO32-+H2O��
��3��E������4.4g����������CO2��4.4g�����ʵ�����0.1mol����ԭ�������̼���ƺ�̼�����Ƶ����ʵ����ֱ���x��y����106x+84y=9.5��x+y=0.1�����x=y=0.05mol������̼���Ƶ�����������
��100%=55.8%��
��4������װ���в�����û�б����յ�CO2�����������ͨ������������ɵ�CO2������ƫ�٣����Ը��ݣ�3����֪��xƫ��̼���Ƶ���������ƫ��
��2��A�����տ����е�CO2�ģ�����ʽΪCO2+2OH-=CO32-+H2O��
��3��E������4.4g����������CO2��4.4g�����ʵ�����0.1mol����ԭ�������̼���ƺ�̼�����Ƶ����ʵ����ֱ���x��y����106x+84y=9.5��x+y=0.1�����x=y=0.05mol������̼���Ƶ�����������
| 5.3 |
| 9.5 |
��4������װ���в�����û�б����յ�CO2�����������ͨ������������ɵ�CO2������ƫ�٣����Ը��ݣ�3����֪��xƫ��̼���Ƶ���������ƫ��
���
�⣺��1������װ��ͼ��֪��ԭ���������̼���η�Ӧ����CO2��Ȼ��ͨ������CO2�����㺬�������������ӷ�������C����ϡ���ᣬE���Ǽ�ʯ�ң����ڿ����к���CO2��ˮ����������F�м�ʯ�ҵ����������յ������������ˮ������������̼���壬��ֹ��E�����ĸ��ţ�
�ʴ�Ϊ��ϡ�����ʯ�ң�����ʯ�ң������յ������������ˮ������������̼���壬��ֹ��E�����ĸ��ţ�
��2��A�����տ����е�CO2�ģ�����ʽΪCO2+2OH-=CO32-+H2O���ʴ�Ϊ��CO2+2OH-=CO32-+H2O��
��3��E������4.4g����������CO2��4.4g�����ʵ�����0.1mol����ԭ�������̼���ƺ�̼�����Ƶ����ʵ����ֱ���x��y����106x+84y=9.5��x+y=0.1�����x=y=0.05mol������̼���Ƶ�����������
��100%=55.8%���ʴ�Ϊ��55.8%��
��4������װ���в�����û�б����յ�CO2�����������ͨ������������ɵ�CO2������ƫ�٣����Ը��ݣ�3����֪��xƫ��̼���Ƶ���������ƫ�ʴ�Ϊ��ƫ��
�ʴ�Ϊ��ϡ�����ʯ�ң�����ʯ�ң������յ������������ˮ������������̼���壬��ֹ��E�����ĸ��ţ�
��2��A�����տ����е�CO2�ģ�����ʽΪCO2+2OH-=CO32-+H2O���ʴ�Ϊ��CO2+2OH-=CO32-+H2O��
��3��E������4.4g����������CO2��4.4g�����ʵ�����0.1mol����ԭ�������̼���ƺ�̼�����Ƶ����ʵ����ֱ���x��y����106x+84y=9.5��x+y=0.1�����x=y=0.05mol������̼���Ƶ�����������
| 5.3 |
| 9.5 |
��4������װ���в�����û�б����յ�CO2�����������ͨ������������ɵ�CO2������ƫ�٣����Ը��ݣ�3����֪��xƫ��̼���Ƶ���������ƫ�ʴ�Ϊ��ƫ��
���������⿼�����ʺ����IJⶨ���漰��ѧʵ�顢��ѧ����ȣ���ȷ�ⶨԭ���ǹؼ������ضԻ���֪ʶ���ۺϿ�����Ǩ��Ӧ�ã��Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
����������Һ�����Ȼ��ƺ͵������ˮ��Һ ��39%���Ҵ���Һ �����ͺ��Ȼ�����Һ���������ϸ����Һ����ȷ���������ǣ�������
| A����Һ����ȡ������ |
| B��������ȡ����Һ |
| C����Һ��������ȡ |
| D����ȡ������Һ |
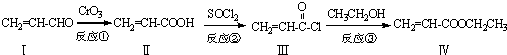
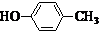 Ҳ���뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ
Ҳ���뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ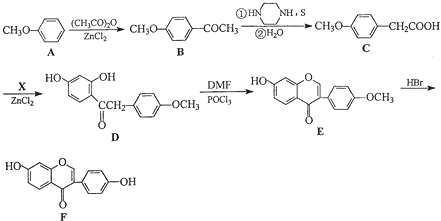
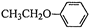 �ͣ�CH3CO��2OΪԭ���Ʊ�ҩ���м���
�ͣ�CH3CO��2OΪԭ���Ʊ�ҩ���м��� �ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�H2C=CH2
�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�H2C=CH2
 �������ڷ�����д���ϳ�·�����̣����Լ���ѡ����ע���ϳ�·�����̵���д��ʽ��������ʾ����
�������ڷ�����д���ϳ�·�����̣����Լ���ѡ����ע���ϳ�·�����̵���д��ʽ��������ʾ����
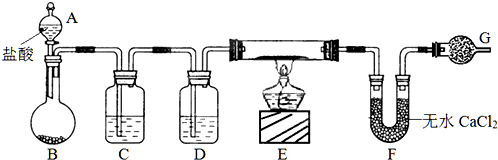
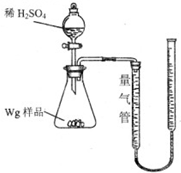 ����ͼ��ʾװ�òⶨFe��Fe2O3�Ļ�����е������������������гֲ�������ȥ������ȡWg��Ʒ������ƿ�У�ͨ����Һ©����������ϡ����ʹ��Ʒ��ȫ�ܽ⣮ʵ��ǰ��������ʼ����ΪamL��ʵ��������ܵ����ն���ΪbmL��
����ͼ��ʾװ�òⶨFe��Fe2O3�Ļ�����е������������������гֲ�������ȥ������ȡWg��Ʒ������ƿ�У�ͨ����Һ©����������ϡ����ʹ��Ʒ��ȫ�ܽ⣮ʵ��ǰ��������ʼ����ΪamL��ʵ��������ܵ����ն���ΪbmL��