��Ŀ����
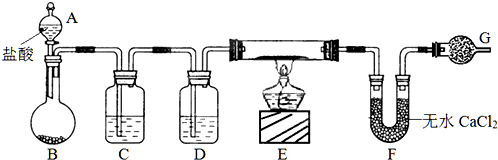
ʵ������CuO��ͭ�۵Ļ���Ҫ�ⶨ����Ʒ���ѳƵ�������Ϊm g��������ͭ����������������ͼ��ʾ����װ����ȡH2������H2��ԭCuO����������Ʒ�����ļ��ٺ���ˮCaCl2�������������ⶨ��Ʒ������ͭ������������
��ش𣺣�1��������װ����Լ���D ��
��2���������ٴ�A�Ļ������͡��ڵ�ȼE���ƾ��ơ�Ӧ���Ƚ��е��� ������ţ���������������֮�仹Ӧ���еIJ����ǣ� ��
��3����ʵ�����в���CuO����ԭΪ��ɫ��Cu2O����ʵ���Ӱ��Ϊ ��ƫ��ƫС����Ӱ�죩��
��4������õ�ԭ����Ϊa g��Ӳ�ʲ����ܣ�E������Ӧ��������Ϊb g��U��ʵ�����������n g�����������ݿ����г���������ͭ����������������ͬ����ʽ������ʽ1�� ������ʽ2�� ��

��ش𣺣�1��������װ����Լ���D
��2���������ٴ�A�Ļ������͡��ڵ�ȼE���ƾ��ơ�Ӧ���Ƚ��е���
��3����ʵ�����в���CuO����ԭΪ��ɫ��Cu2O����ʵ���Ӱ��Ϊ
��4������õ�ԭ����Ϊa g��Ӳ�ʲ����ܣ�E������Ӧ��������Ϊb g��U��ʵ�����������n g�����������ݿ����г���������ͭ����������������ͬ����ʽ������ʽ1��
���㣺̽�����ʵ���ɻ�������ʵĺ���,ͭ����������Ҫ���������Ҫ����
ר�⣺������Ҫ�Ľ������仯����
��������ȡH2������H2��ԭCuO����������Ʒ�����ļ��ٺ���ˮCaCl2�������������ⶨ��Ʒ������ͭ����������������Aװ������ȡ������װ�ã��Ƶõ������к���HCl���ʣ�ͨ��Bװ���еı���ʳ��ˮ�����գ�C�Ǹ���������װ�ã�����C��ʢ�ŵ���Ũ���ᣬE��������ԭ����ͭװ�ã�F�е���ˮ�Ȼ������յ���������ԭ����ͭ�õ���ˮ������װ���Ƿ�ֹ������ˮ�ĸ��ŵģ��ݴ˻ش��жϣ�
���
�⣺��ȡH2������H2��ԭCuO����������Ʒ�����ļ��ٺ���ˮCaCl2�������������ⶨ��Ʒ������ͭ����������������Aװ������ȡ������װ�ã��Ƶõ������к���HCl���ʣ�ͨ��Bװ���еı���ʳ��ˮ�����գ�C�Ǹ���������װ�ã�����C��ʢ�ŵ���Ũ���ᣬE��������ԭ����ͭװ�ã�F�е���ˮ�Ȼ������յ���������ԭ����ͭ�õ���ˮ������װ���Ƿ�ֹ������ˮ�ĸ��ŵģ�
��1��C�Ǹ���������װ�ã�C��ʢ�ŵ���Ũ���ᣬ�ʴ�Ϊ��Ũ���
��2���������ٴ�A�Ļ������������������ֽ�װ���еĿ����Լ�ˮ�����ž�����������������ȼE���ƾ��ơ������Լ�Сʵ�����ʴ�Ϊ���٣�
�ռ�G����������鴿�ȣ�
��3����ʵ�����в���CuO����ԭΪ��ɫ��Cu2O����E�й����С������ƫС������ͭ����������ƫС���ʴ�Ϊ��ƫС��
��4������õ�ԭ����Ϊa g��Ӳ�ʲ����ܣ�E������Ӧ��������Ϊb g��
���ݹ���������������CuO+H2
Cu+H2O ����������С
80 64 16
5��a-b��g ��a-b��g
��������ͭ����������=
��U��ʵ�����������n g��������ˮ��������ng����
CuO+H2
Cu+H2O
80 18
ng
��ʱ����ͭ������������
=
��
�ʴ�Ϊ��
��
��
��1��C�Ǹ���������װ�ã�C��ʢ�ŵ���Ũ���ᣬ�ʴ�Ϊ��Ũ���
��2���������ٴ�A�Ļ������������������ֽ�װ���еĿ����Լ�ˮ�����ž�����������������ȼE���ƾ��ơ������Լ�Сʵ�����ʴ�Ϊ���٣�
�ռ�G����������鴿�ȣ�
��3����ʵ�����в���CuO����ԭΪ��ɫ��Cu2O����E�й����С������ƫС������ͭ����������ƫС���ʴ�Ϊ��ƫС��
��4������õ�ԭ����Ϊa g��Ӳ�ʲ����ܣ�E������Ӧ��������Ϊb g��
���ݹ���������������CuO+H2
| ||
80 64 16
5��a-b��g ��a-b��g
��������ͭ����������=
| 5(a-b) |
| m |
CuO+H2
| ||
80 18
ng
��ʱ����ͭ������������
| 80n |
| 18m |
| 40n |
| 9m |
�ʴ�Ϊ��
| 5(a-b) |
| m |
| 40n |
| 9m |
�����������ǹ������ʵ�����Լ������IJⶨ��Ŀ������Ĺؼ�������ʵ��ԭ���Լ�ʵ��Ľ����Ӱ�����أ��ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
�����£���VmLŨ��Ϊcmol/L��CH3COOH��Һ����μ���2VmL��ͬ���ʵ���Ũ�ȵ�NaOH��Һ�������й�������ȷ���ǣ�������
| A��ԭCH3COOH��Һ��c��H+����NaOH��Һ��c��OH-����� |
| B���˹�����Һ��ˮ�ĵ���̶���������С����Һ��pH���� |
| C������VmLʱ����Һ������ |
| D������2VmLʱ����Һ��c��CH3COO-��+c��CH3COOH��=c��OH-��-c��H+�� |
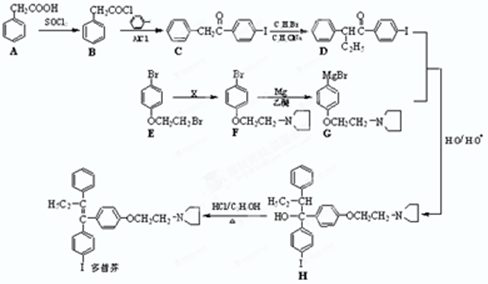
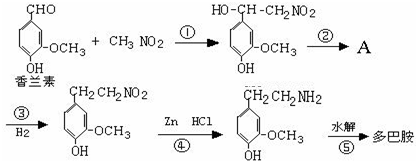
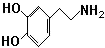
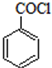 ���Ǻϳ�ҩƷ����Ҫ�м��壮��д���Ա������ѡ���ȩΪԭ���Ʊ��������ȵĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�
���Ǻϳ�ҩƷ����Ҫ�м��壮��д���Ա������ѡ���ȩΪԭ���Ʊ��������ȵĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�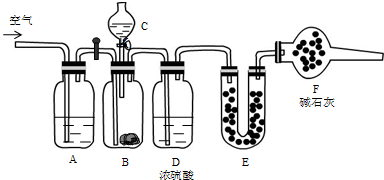
 ��
��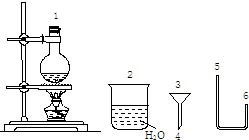 ������500mL0.2mol/LNa2CO3��Һ���ش��������⣺
������500mL0.2mol/LNa2CO3��Һ���ش��������⣺